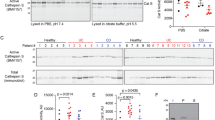
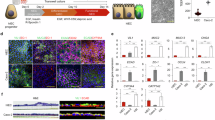
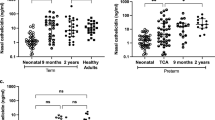
Abstract
Cathelicidins, a family of host defense peptides, are highly expressed during infection, inflammation and wound healing. These peptides not only have broad-spectrum antimicrobial activities, but also modulate inflammation by altering cytokine response and chemoattraction of inflammatory cells in diseased tissues. In this connection, a mouse cathelicidin has been demonstrated to prevent inflammation in the colon through enhancing mucus production and reducing production of pro-inflammatory cytokines. In addition, cathelicidins promote wound healing through stimulation of re-epithelialization and angiogenesis at injured tissues. In an animal model of gastric ulceration, the rat cathelicidin promotes ulcer healing by inducing proliferation of gastric epithelial cells both in vitro and in vivo. In conclusion, cathelicidins represent an important group of effector molecules in the innate immune system that operates a complex integration of inflammation and tissue repair in the gastrointestinal mucosa and other organs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DSS:
-
dextran sulfate sodium
- EGFR:
-
epidermal growth factor receptors
- FPRL1:
-
formyl peptide receptor-like 1
- IL:
-
interleukin
- HB-EGF:
-
heparin-binding-EGF-like growth factor
- MAP:
-
mitogen-activated protein
- mCRAMP:
-
mouse cathelicidin
- MMP:
-
matrix metalloproteinases
- rCRAMP:
-
rat cathelicidin
- TGFα:
-
transforming growth factor α
- TNF:
-
tumor necrosis factor
- UC:
-
ulcerative colitis
References
Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, et al. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer 2010. doi: 10.1002/ijc.25489
Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000; 96: 3086–93.
Bals R, Wang X, Zasloff M, Wilson JM . The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 1998; 95: 9541–6.
Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 2003; 125: 1613–25.
Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF . Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002; 70: 953–63.
Gallo RL, Nizet V . Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr Allergy Asthma Rep 2003; 3: 402–9.
Huang HJ, Ross CR, Blecha F . Chemoattractant properties of PR-39, a neutrophil antibacterial peptide. J Leukoc Biol 1997; 61: 624–9.
De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000; 192: 1069–74.
Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003; 120: 379–89.
Yang YH, Wu WK, Tai EK, Wong HP, Lam EK, So WH, et al. The cationic host defense peptide rCRAMP promotes gastric ulcer healing in rats. J Pharmacol Exp Ther 2006; 318: 547–54.
Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997; 272: 15258–63.
Kim ST, Cha HE, Kim DY, Han GC, Chung YS, Lee YJ, et al. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol 2003; 123: 81–5.
Verbanac D, Zanetti M, Romeo D . Chemotactic and protease-inhibiting activities of antibiotic peptide precursors. FEBS Lett 1993; 317: 255–8.
Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 2002; 106: 20–6.
Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D . Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol 2005; 174: 6257–65.
Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J Immunol 2001; 167: 3329–38.
Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 2003; 171: 6690–6.
Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE . The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol 2002; 169: 3883–91.
Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol 2005; 174: 4271–8.
Elssner A, Duncan M, Gavrilin M, Wewers MD . A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol 2004; 172: 4987–94.
Osborne BA . Apoptosis and the maintenance of homoeostasis in the immune system. Curr Opin Immunol 1996; 8: 245–54.
Risso A, Zanetti M, Gennaro R . Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell Immunol 1998; 189: 107–15.
Kim WD . Lung mucus: a clinician's view. Eur Respir J 1997; 10: 1914–7.
Corfield AP, Myerscough N, Bradfield N, Corfield Cdo A, Gough M, Clamp JR, et al. Colonic mucins in ulcerative colitis: evidence for loss of sulfation. Glycoconj J 1996; 13: 809–22.
Kim YS, Gum J, Jr., Brockhausen I . Mucin glycoproteins in neoplasia. Glycoconj J 1996; 13: 693–707.
Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994; 35: 353–9.
Enss ML, Cornberg M, Wagner S, Gebert A, Henrichs M, Eisenblatter R, et al. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res 2000; 49: 162–9.
Tai EK, Wu WK, Wong HP, Lam EK, Yu L, Cho CH . A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med (Maywood) 2007; 232: 799–808.
Hayashi T, Ishida T, Motoya S, Itoh F, Takahashi T, Hinoda Y, et al. Mucins and immune reactions to mucins in ulcerative colitis. Digestion 2001; 63: 28–31.
Iwashita J, Sato Y, Sugaya H, Takahashi N, Sasaki H, Abe T . mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-α through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol 2003; 81: 275–82.
Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, et al. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem 2008; 104: 251–8.
Brandenburg LO, Seyferth S, Wruck CJ, Koch T, Rosenstiel P, Lucius R, et al. Involvement of Phospholipase D 1 and 2 in the subcellular localization and activity of formyl-peptide-receptors in the human colonic cell line HT29. Mol Membr Biol 2009; 26: 371–83.
Selzner N, Selzner M, Graf R, Ungethuem U, Fitz JG, Clavien PA . Water induces autocrine stimulation of tumor cell killing through ATP release and P2 receptor binding. Cell Death Differ 2004;11 Suppl 2: S172–80.
Falanga V . Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736–43.
Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol 2001; 117: 91–7.
Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003; 120: 379–89.
Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 2003; 111: 1665–72.
Li J, Post M, Volk R, Gao Y, Li M, Metais C, et al. PR39, a peptide regulator of angiogenesis. Nat Med 2000; 6: 49–55.
Chon JH, Houston MM, Xu C, Chaikof EL . PR-39 coordinates changes in vascular smooth muscle cell adhesive strength and locomotion by modulating cell surface heparan sulfate-matrix interactions. J Cell Physiol 2001; 189: 133–43.
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A 1994; 91: 11035–9.
Woodley DT, Chen JD, Kim JP, Sarret Y, Iwasaki T, Kim YH, et al. Re-epithelialization. Human keratinocyte locomotion. Dermatol Clin 1993; 11: 641–6.
Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 2005; 175: 4662–8.
Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 2005; 289: L842–8.
Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 2005; 289: L842–8.
Acknowledgements
This work is partially supported by the Downstream Development Seed Fund, The Chinese University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Wong, C., Li, Z. et al. Cathelicidins in inflammation and tissue repair: Potential therapeutic applications for gastrointestinal disorders. Acta Pharmacol Sin 31, 1118–1122 (2010). https://doi.org/10.1038/aps.2010.117
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.117
Keywords
This article is cited by
-
The absence of murine cathelicidin-related antimicrobial peptide impacts host responses enhancing Salmonella enterica serovar Typhimurium infection
Gut Pathogens (2020)
-
Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp−/− mice
Gene Therapy (2013)
-
Cathelicidin gene therapy: a new therapeutic option in ulcerative colitis and beyond?
Gene Therapy (2013)
-
Cathelicidin protects against Helicobacter pylori colonization and the associated gastritis in mice
Gene Therapy (2013)