Abstract
Aim:
To ascertain the effects of erlotinib on CYP3A, to investigate the amplitude and kinetics of erlotinib-mediated inhibition of seven major CYP isoforms in human liver microsomes (HLMs) for evaluating the magnitude of erlotinib in drug-drug interaction in vivo.
Methods:
The activities of 7 major CYP isoforms (CYP1A2, CYP2A6, CYP3A, CYP2C9, CYP2D6, CYP2C8, and CYP2E1) were assessed in HLMs using HPLC or UFLC analysis. A two-step incubation method was used to examine the time-dependent inhibition of erlotinib on CYP3A.
Results:
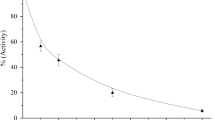
The activity of CYP2C8 was inhibited with an IC50 value of 6.17±2.0 μmol/L. Erlotinib stimulated the midazolam 1′-hydroxy reaction, but inhibited the formation of 6β-hydroxytestosterone and oxidized nifedipine. Inhibition of CYP3A by erlotinib was substrate-dependent: the IC50 values for inhibiting testosterone 6β-hydroxylation and nifedipine metabolism were 31.3±8.0 and 20.5±5.3 μmol/L, respectively. Erlotinib also exhibited the time-dependent inhibition on CYP3A, regardless of the probe substrate used: the value of KI and kinact were 6.3 μmol/L and 0.035 min−1 for midazolam; 9.0 μmol/L and 0.045 min−1 for testosterone; and 10.1 μmol/L and 0.058 min−1 for nifedipine.
Conclusion:
The inhibition of CYP3A by erlotinib was substrate-dependent, while its time-dependent inhibition on CYP3A was substrate-independent. The time-dependent inhibition of CYP3A may be a possible cause of drug-drug interaction, suggesting that attention should be paid to the evaluation of erlotinib's safety, especially in the context of combination therapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ciardiello F, Tortora G . A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin Cancer Res 2001; 7: 2958–70.
Smith J . Erlotinib: Small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clin Ther 2005; 27: 1513–34.
Shepherd FA, Pereira JR, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123–32.
Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R . FDA drug approval summary: Erlotinib (Tarceva (R)) tablets. Oncologist 2005; 10: 461–6.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol 2007; 25: 1960–6.
Vasey PA, Gore M, Wilson R, Rustin G, Gabra H, Guastalla JP, et al. A phase Ib trial of docetaxel, carboplatin and erlotinib in ovarian, fallopian tube and primary peritoneal cancers. Br J Cancer 2008; 98: 1774–80.
Lin CC, Calvo E, Papadopoulos KP, Patnaik A, Sarantopoulos J, Mita AC, et al. Phase I study of cetuximab, erlotinib, and bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol 2009; 63: 1065–71.
Thomas F, Rochaix P, White-Koning M, Hennebelle I, Sarini J, Benlyazid A, et al. Population pharmacokinetics of erlotinib and its pharmacokinetic/pharmacodynamic relationships in head and neck squamous cell carcinoma. Eur J Cancer 2009; 45: 2316–23.
Gridelli C, Rossi A, Malone P, Colantuoni G, Del Gaizo F, Ferrara C, et al. Erlotinib in non-small-cell lung cancer. Expert Opin Pharmacother 2007; 8: 2579–92.
Schwiertz V, Bertin C, Henry A, Charpiat B . Number and nature of drug interactions concerning antineoplastic drugs. Bull Cancer 2007; 94: 477–82.
Patnaik A, Wood D, Tolcher AW, Hamilton M, Kreisberg JI, Hammond LA, et al. Phase 1, pharmacokinetic, and biological study of erlotinib in combination with paclitaxel and carboplatin in patients with advanced solid tumors. Clin Cancer Res 2006; 12: 7406–13.
Grenader T, Gipps M, Shavit L, Gabizon A . Significant drug interaction: Phenytoin toxicity due to erlotinib. Lung Cancer 2007; 57: 404–6.
Veeraputhiran M, Sundermeyer M . Rhabdomyolysis resulting from pharmacologic interaction between erlotinib and simvastatin. Clin Lung Cancer 2008; 9: 232–34.
Ling J, Johnson KA, Miao Z, Rakhit A, Pantze MP, Hamilton M, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos 2006; 34: 420–6.
Li J, Zhao M, He P, Hidalgo M, Baker SD . Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res 2007; 13: 3731–7.
Harmsen S, Meijerman I, Beijnen JH, Schellens JHM . Nuclear receptor mediated induction of cytochrome P450 3A4 by anticancer drugs: a key role for the pregnane X receptor. Cancer Chemother Pharmacol 2009; 64: 35–43.
Liu Y, Ramirez J, House L, Ratain MJ . Comparison of the drug-drug interactions potential of erlotinib and gefitinib via inhibition of UDP-glucuronosyltransferases. Drug Metab Dispos 2010; 38: 32–9.
Zhang YY, Liu Y, Zhang JW, Ge GB, Liu HX, Wang LM, et al. C-7 configuration as one of determinants in taxanes metabolism by human cytochrome P450 enzymes. Xenobiotica 2009; 39: 903–14.
Liang SC, Ge GB, Liu HX, Zhang YY, Wang LM, Zhang JW, et al. Identification and characterization of human udp-glucuronosyltransferases responsible for the in vitro glucuronidation of daphnetin. Drug Metab Dispos 2010; 38: 973–80.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ . Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–75.
Liu Y, Ma H, Zhang JW, Deng MC, Yang L . Influence of ginsenoside Rh-1 and F-1 on human cytochrome P450 enzymes. Planta Med 2006; 72: 126–31.
Zhang JW, Liu Y, Li W, Hao DC, Yang L . Inhibitory effect of medroxyprogesterone acetate on human liver cytochrome P450 enzymes. Eur J Clin Pharmacol 2006; 62: 497–502.
Dong PP, Ge GB, Zhang YY, Ai CZ, Li GH, Zhu LL, et al. Quantitative structure-retention relationship studies for taxanes including epimers and isomeric metabolites in ultra fast liquid chromatography. J Chromatogr A 2009; 1216: 7055–62.
Liu YT, Hao K, Liu XQ, Wang GJ . Metabolism and metabolic inhibition of gambogic acid in rat liver microsomes. Acta Pharmacol Sin 2006; 27: 1253–58.
Fang ZZ, Zhang YY, Ge GB, Huo H, Liang SC, Yang L . Time-dependent inhibition (TDI) of CYP3A4 and CYP2C9 by noscapine potentially explains clinical noscapine-warfarin interaction. Br J Clin Pharmacol 2010; 69: 193–9.
Korzekwa KR, Krishnamachary N, Shou M, Ogai A, Parise RA, Rettie AE, et al. Evaluation of atypical cytochrome P450 kinetics with two-substrate models: Evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry 1998; 37: 4137–47.
Obach RS, Walsky RL, Venkatakrishnan K . Mechanism-based inactivation of human cytochrome P450 enzymes and the prediction of drug-drug interactions. Drug Metab Dispos 2007; 35: 246–55.
Austin RP, Barton P, Cockroft SL, Wenlock MC, Riley RJ . The influence of nonspecific microsomal binding on apparent intrinsic clearance, and its prediction from physicochemical properties. Drug Metab Dispos 2002; 30: 1497–503.
Brown HS, Ito K, Galetin A, Houston JB . Prediction of in vivo drug-drug interactions from in vitro data: impact of incorporating parallel pathways of drug elimination and inhibitor absorption rate constant. Br J Clin Pharmacol 2005; 60: 508–18.
Fahmi OA, Hurst S, Plowchalk D, Cook J, Guo F, Youdim K, et al. Comparison of different algorithms for predicting clinical drug-drug interactions, based on the use of cyp3a4 in vitro data: predictions of compounds as precipitants of interaction. Drug Metab Dispos 2009; 37: 1658–66.
Yamamoto N, Horiike A, Fujisaka Y, Murakami H, Shimoyama T, Yamada Y, et al. Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor Ro50-8231 (erlotinib) in Japanese patients with solid tumors. Cancer Chemother Pharmacol 2008; 61: 489–96.
Hutzler JM, Kolwankar D, Hummel MA, Tracy TS . Activation of CYP2C9-mediated metabolism by a series of dapsone analogs: Kinetics and structural requirements. Drug Metab Dispos 2002; 30: 1194–200.
Rendic S . Summary of information on human CYP enzymes: Human P450 metabolism data. Drug Metab Rev 2002; 34: 83–448.
van Erp NP, Gelderblom H, Guchelaar HJ . Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009; 35: 692–706.
Wang RW, Newton DJ, Liu N, Atkins WM, Lu AYH . Human cytochrome P-450 3A4: In vitro drug-drug interaction patterns are substrate-dependent. Drug Metab Dispos 2000; 28: 360–6.
Schwab GE, Raucy JL, Johnson EF . Modulation of rabbit and human hepatic cytochrome-p-450-catalyzed steroid hydroxylations by alpha-naphthoflavone. Mol Pharmacol 1988; 33: 493–9.
Yun CH, Wood M, Wood AJJ, Guengerich FP . Identification of the pharmacogenetic determinants of alfentanil metabolism-cytochrome p-450-3a4 — an explanation of the variable elimination clearance. Anesthesiology 1992; 77: 467–74.
Hao M, Zhao YQ, Chen PZ, Huang H, Liu H, Jiang HL, et al. Structure-activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing p450 enzymes. PLoS One 2008; 3: e2697.
Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, et al. Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 2004; 305: 683–6.
Tracy TS . Atypical enzyme kinetics: Their effect on in vitro-in vivo pharmacokinetic predictions and drug interactions. Curr Drug Metab 2003; 4: 341–6.
Nomeir AA, Ruegg C, Shoemaker M, Favreau LV, Palamanda JR, Silber P, et al. Inhibition of CYP3A4 in a rapid microtiter plate assay using recombinant enzyme and in human liver microsomes using conventional substrates. Drug Metab Dispos 2001; 29: 748–53.
Grimm SW, Einolf HJ, Hall SD, He K, Lim HK, Ling KHJ, et al. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of america. Drug Metab Dispos 2009; 37: 1355–70.
Henshall J, Galetin A, Harrison A, Houston JB . Comparative analysis of CYP3A heteroactivation by steroid hormones and flavonoids in different in vitro systems and potential in vivo implications. Drug Metab Dispos 2008; 36: 1332–40.
Gibbs MA, Thummel KE, Shen DD, Kunze KL . Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: Comparison of K–I values and impact of CYP3A5 expression. Drug Metab Dispos 1999; 27: 180–7.
McConn DJ, Lin YS, Allen K, Kunze KL, Thummel KE . Differences in the inhibition of cytochromes P450 3A4 and 3A5 by metabolite-inhibitor complex-forming drugs. Drug Metab Dispos 2004; 32: 1083–91.
Li XH, Kamenecka TM, Cameron MD . Cytochrome P450-mediated bioactivation of the epidermal growth factor receptor inhibitor erlotinib to a reactive electrophile. Drug Metab Dispos 2010; 38: 1238–45.
Li X, Kamenecka TM, Cameron MD . Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol 2009; 22: 1736–42.
Li XH, He YJ, Ruiz CH, Koenig M, Cameron MD . Characterization of dasatinib and its structural analogs as cyp3a4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metab Dispos 2009; 37: 1242–50.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 30630075, 30772608, 30973590, and 81072698), the National Key Technology R&D Program in the 11th Five-year Plan of China (No 2008ZX10002-019) and the National Science & Technology Pillar Program of China (No 2009BADB9B02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Pp., Fang, Zz., Zhang, Yy. et al. Substrate-dependent modulation of the catalytic activity of CYP3A by erlotinib. Acta Pharmacol Sin 32, 399–407 (2011). https://doi.org/10.1038/aps.2010.218
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.218
Keywords
This article is cited by
-
Interaction between phytotherapy and oral anticancer agents: prospective study and literature review
Medical Oncology (2019)
-
Evaluation of Time Dependent Inhibition Assays for Marketed Oncology Drugs: Comparison of Human Hepatocytes and Liver Microsomes in the Presence and Absence of Human Plasma
Pharmaceutical Research (2016)
-
Pharmacokinetic Properties of Two Erlotinib 150 mg Formulations with a Genetic Effect Evaluation in Healthy Korean Subjects
Clinical Drug Investigation (2015)
-
Exposure–Toxicity Relationship of Sorafenib in Japanese Patients with Renal Cell Carcinoma and Hepatocellular Carcinoma
Clinical Pharmacokinetics (2014)
-
Population Pharmacokinetics/Pharmacodynamics of Erlotinib and Pharmacogenomic Analysis of Plasma and Cerebrospinal Fluid Drug Concentrations in Japanese Patients with Non-Small Cell Lung Cancer
Clinical Pharmacokinetics (2013)