Abstract
Aim:
This study investigated the effect of intragastrically administered melatonin on intestinal mucosal permeability induced by diclofenac in mice.
Methods:
Intestinal mucosal permeability was induced in mice by intragastric administration of diclofenac (2.5 mg/kg). Melatonin was given intragastrically (10 mg/kg) once per day for 3 d after diclofenac administration. The small intestine was examined macroscopically and microscopically for pathologic injury to the intestinal mucosa. Intestinal mucosal permeability was evaluated by Evans blue and FITC-dextran methods. Mitochondrial functional parameters, including mitochondrial membrane potential, mitochondrial ATPase and succinate dehydrogenase (SDH) activity, were assessed. The malondialdehyde (MDA) and myeloperoxidase (MPO) levels were determined from small intestinal mucosal homogenates.
Results:
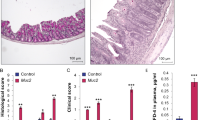
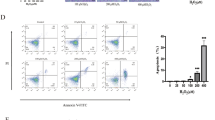
As compared with control mice, the permeability, pathologic score, MDA and MPO levels and ulceration of the intestinal mucosa were increased significantly by diclofenac treatment, and a broadened junctional complex and enlarged intercellular space were observed by transmission electron microscopy (TEM). Melatonin treatment significantly reduced the intestinal mucosal permeability, pathologic score, MDA, and MPO levels and ulceration of the intestinal mucosa. By TEM, the small intestine villus morphology and intercellular spaces were nearly normal in melatonin-treated mice. At the level of the mitochondria, melatonin treatment significantly restored the activities of ATPase and SDH.
Conclusion:
The intestinal damage and increased intestinal permeability induced by diclofenac in mice was limited by melatonin; moreover, melatonin preserved several aspects of mitochondrial function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fortun PJ, Hawkey CJ . Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol 2005; 21: 169–75.
Arrieta MC, Bistritz L, Meddings JB . Alterations in intestinal permeability. Gut 2006; 55: 1512–20.
Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R, et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut 1998; 43: 506–11.
Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut 1999; 45: 362–6.
Maiden L . Capsule endoscopic diagnosis of nonsteroidal anti-inflammatory drug-induced enteropathy. J Gastroenterol 2009; 44: 64–71.
van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, et al. Glutamine and the preservation of gut integrity. Lancet 1993; 341: 1363–5.
Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ . A review of the multiple action of melatonin on the immune system. Endocrine 2005; 27: 189–200.
Dobocovich M, Mankowsha M . Functimal MP1 and MP2 melatonin receptors in mammals. Endocrine 2005; 27: 101–10.
Bubenik GA . Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signals Recept 2001; 10: 350–66.
Huether G . Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann NY Acad Sci 1994; 719: 146–58.
Cabeza J, Motilva V, Martín MJ, de la Lastra CA . Mechanisms involved in gastric protection of melatonin against oxidant stress by ischemia-reperfusion in rats. Life Sci 2001; 68: 1405–15.
Bilici D, Süleyman H, Banoğlu ZN, Kiziltunç A, Avci B, Ciftçioğlu A, et al. Melatonin prevents ethanol-induced gastric mucosal damage possibly due to its antioxidant effect. Dig Dis Sci 2002; 47: 856–61.
Alarcón de la Lastra C, Motilva V, Martín MJ, Nieto A, Barranco MD, Cabeza J, et al. Protective effect of melatonin on indomethacin-induced gastric injury in rats. J Pineal Res 1999; 26: 101–7.
Konturek PC, Konturek SJ, Ketal C . Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in human. J Pineal Res 2004; 48: 318–23.
Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC . Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res 1993; 14: 151–68.
Reiter RJ . Melatonin: lowering the high price of free radicals. News Physiol Sci 2000; 15: 246–50.
Reiter RJ, Paredes SD, Manchester LC, Tan DX . Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol 2009; 44: 175–200.
Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, et al. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res 2009; 46: 314–23.
Atchison CR, West AB, Balakumaran A, Hargus SJ, Pohl LR, Daiker DH, et al. Drug enterocyte adducts: possible causal factor for diclofenac enteropathy in rats. Gastroenterology 2000; 119: 1537–47.
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN . Intestinal mucosal lesion in low-flow states. l. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970; 101: 478–83.
Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC . Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 2002; 92: 1750–61.
Lange S, Delbro DS, Jennische E . Evans blue permeation of intestinal mucosa in the rat. Scand J Gastroenterol 1994; 29: 38–46.
Lowry OH, Rosebrough NJ, Farr A L, Randall RJ . Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–75.
Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 1997; 41: 344–53.
Schneider WC, Hogeboom KEO . Intracellular distribution of enzymes. V. Further studies on the distribution of cytochrome c in rat liver homogenates. J Biol Chem 1950; 183: 123–8.
Emaus RK, Grunwald R, Lemaster JJ . Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria. Biochim Biophys Acta 1986; 850: 436–48.
Hernández-Muñoz R, Díaz-Muñoz M, Chagoya de Sánchez V . Effects of adenosine administration on the function and membrane composition of liver mitochondria in carbon tetrachloride-induced cirrhosis. Arch Biochem Biophys 1992; 294: 160–7.
Veeger C, Vartanian DV, Zeylemaker WP . Succinate dehydrogenase. In: Methods in enzymology. Lowenstein JM, editors. New York: Academic Press; 1969. p 81–90.
Parmar DV, Ahmed G, Khandkar MA, Katyare SS . Mitochondrial ATPase: a target for paracetamol-induced hepatotoxicity. Eur J Pharmacol 1995; 293: 225–9.
Davies NM, Saleh JY, Skjodt NM . Detection and prevention of NSAID-induced enteropathy. J Pharm Pharm Sci 2000; 3: 137–55.
Bjarnason I, Zanelli G, Smith T, Prouse P, Williams P, Smethurst P, et al. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology 1987; 93: 480–9.
Bjarnason I, Takeuchi K . Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol 2009; 44: 23–9.
Ozturk H, Oztürk H, Yagmur Y, Uzunlar AK . Effects of melatonin administration on intestinal adaptive response after massive bowel resection in rats. Dig Dis Sci 2006; 51: 333–7.
Cabeza J, Alarcón-de-la-Lastra C, Jiménez D, Martín MJ, Motilva V . Melatonin modulates the effects of gastric injury in rats: role of prostaglandins and nitric oxide. Neurosignals 2003; 12: 71–7.
Bandyopadhyay D, Bandyopadhyay A, Das PK, Reiter RJ . Melatonin protects against gastric ulceration and increases the efficacy of ranitidine and omeprazole in reducing gastric damage. J Pineal Res 2002; 33: 1–7.
Hardeland R, Tan DX, Reiter RJ . Kynuramines, metabolites of melatonin and other indoles:the resurrection of an almost forgotton class of biogenic amines. J Pineal Res 2009; 47: 109–24.
Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ . Oxidative stress of the newborn in the pre-and postnatal period and the clinical utility of melatonin. J Pineal Res 2009; 44: 128–39.
Pablos MI, Reiter RJ, Ortiz GG, Guerrero JM, Agapito MT, Chuang JI, et al. Rhythms of glutathione peroxidase and glutathione reductase in brain of chick and their inhibition by light. Neurochem Int 1998; 32: 69–75.
Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter RJ, Yang CM, et al. Visualization of melatonin's multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. J Pineal Res 2010; 48: 20–38.
Kim SH, Lee SM . Cytoprotective effects of melatonin against necrosis and apoptosis induced by ischemia/reperfusion injury in rat liver. J Pineal Res 2008; 44: 165–71.
Okatani Y, Wakatsuki A, Reiter RJ, Enzan H, Miyahara Y . Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver. Eur J Pharmacol 2003; 469: 145–52.
Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF . Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J 2004; 18: 869–71.
López A, García JA, Escames G, Venegas C, Ortiz F, López LC, et al. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 2009; 46: 188–98.
Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D . Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol 2002; 34: 348–57.
Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H . Mitochondrial regulation by melatonin and its metabolites. Adv Exp Med Biol 2003; 527: 549–57.
Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, et al. Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res 2001; 30: 65–74.
Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM . Melatonin, cardiolirin and mitochondrial bioenergetics in health and disease. J Pineal Res 2010; 48: 297–310.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mei, Q., Diao, L., Xu, Jm. et al. A protective effect of melatonin on intestinal permeability is induced by diclofenac via regulation of mitochondrial function in mice. Acta Pharmacol Sin 32, 495–502 (2011). https://doi.org/10.1038/aps.2010.225
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.225