Abstract
Aim:
To investigate the effects of diltiazem, an L-type calcium channel blocker, and propafenone, a sodium channel blocker, on the inactivation and recovery kinetics of fKv1.4, a potassium channel that generates the cardiac transient outward potassium current.
Methods:
The cRNA for fKv1.4ΔN, an N-terminal deleted mutant of the ferret Kv1.4 potassium channel, was injected into Xenopus oocytes to express the fKv1.4ΔN channel in these cells. Currents were recorded using a two electrode voltage clamp technique.
Results:
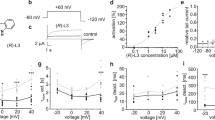
Diltiazem (10 to 1000 μmol/L) inhibited the fKv1.4ΔN channel in a frequency-dependent, voltage-dependent, and concentration-dependent manner, suggesting an open channel block. The IC50 was 241.04±23.06 μmol/L for the fKv1.4ΔN channel (at +50 mV), and propafenone (10 to 500 μmol/L) showed a similar effect (IC50=103.68±10.13 μmol/L). After application of diltiazem and propafenone, fKv1.4ΔN inactivation was bi-exponential, with a faster drug-induced inactivation and a slower C-type inactivation. Diltiazem increased the C-type inactivation rate and slowed recovery in fKv1.4ΔN channels. However, propafenone had no effect on either the slow inactivation time constant or the recovery.
Conclusion:
Diltiazem and propafenone accelerate the inactivation of the Kv1.4ΔN channel by binding to the open state of the channel. Unlike propafenone, diltiazem slows the recovery of the Kv1.4ΔN channel.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Campbell DL, Rasmusson RL, Qu Y, Strauss HC . The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol 1993; 101: 60–6.
Greenstein JL, Wu R, Po S, Tomaselli GF, Winslow RL . Role of the calcium-independent transient outward current Ito1 in shaping action potential morphology and duration. Circ Res 2000; 87: 1026–33.
Wettwer E, Amos GJ, Posival H, Ravens U . Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res 1994; 75: 473–82.
Holmgren M, Jurman ME, Yellen G . N-type inactivation and the S4-S5 region of the Shaker K+ channel. J Gen Physiol 1996; 108: 195–206.
Hoshi T, Zagotta WN, Aldrich RW . Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 1990; 250: 533–8.
Isacoff EY, Jan YN, Jan LY . Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature 1991; 353: 86–90.
Jerng HH, Covarrubias M . K+ channel inactivation mediated by the concerted action of the cytoplasmic N- and C-terminal domains. Biophys J 1997; 72: 163–74.
Lopez GA, Jan YN, Jan LY . Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature 1994; 367: 179–82.
Zagotta WN, Hoshi T, and Aldrich RW . Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 1990; 250: 568–71.
Liu Y, Jurman ME, Yellen G . Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron 1996; 16: 859–67.
Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC . C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol 1995; 489: 709–21.
Kumar S, Hall RJ . Drug treatment of stable angina pectoris in the elderly: defining the place of calcium channel antagonists. Drugs Aging 2003; 20: 805–15.
Hohnloser SH, Kuck KH, Lilienthal J . Rhythm or rate control in atrial fibrillation -pharmacological intervention in atrial fibrillation (PIAF): a randomised trial. Lancet 2000; 356: 1789–94.
Gorenek B, Cavusoglu Y, Goktekin O, Birdane A, Kudaiberdieva G, Ata N, et al. Amiodarone versus sotalol and propafenone for prevention of immediate recurrence of atrial fibrillation after internal cardioversion: importance of P wave analysis. Int J Cardiol 2006; 106: 268–9.
Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, et al. Pharmacological characterization of five cloned voltage gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol 1994; 45: 1227–34.
Rolf S, Haverkamp W, Borggrefe M, Musshoff U, Eckardt L, Mergenthaler J, et al. Effects of antiarrhythmic drugs on cloned cardiac voltage-gated potassium channels expressed in Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol 2000; 362: 22–31.
Caballero R, Gómez R, Núñez L, Moreno I, Tamargo J, Delpón E . Diltiazem inhibits hKv1.5 and Kv4.3 currents at therapeutic concentrations. Cardiovasc Res 2004; 64: 457–66.
Xu L, Huang C, Chen J, Jiang X, Li X, Bett GC, et al. Effect of amiodarone on Kv1.4 channel C-type inactivation: comparison of its effects with those induced by propafenone and verapamil. Pharmazie 2008; 63: 475–9.
Comer MB, Campbell DL, Rasmusson RL, Lamson DR, Morales MJ, Zhang Y, et al. Cloning and characterization of an Ito-like potassium channel from ferret ventricle. Am J Physiol 1994; 267: H1383–95.
Wang S, Morales MJ, Qu YJ, Bett GC, Strauss HC, Rasmusson RL . Kv1.4 channel block by quinidine: evidence for a drug induced allosteric effect. J Physiol 2003; 546: 387–401.
Madeja M, Leicher T, Friederich P, Punke MA, Haverkamp W, Musshoff U, et al. Molecular site of action of the antiarrhythmic drug propafenone at the voltage-operated potassium channel Kv2.1. Mol Pharmacol 2003; 63: 547–56.
Arias C, González T, Moreno I, Caballero R, Delpón E, Tamargo J, et al. Effects of propafenone and its main metabolite, 5-hydroxypropafenone, on hERG channels. Cardiovasc Res 2003; 57: 660–9.
Franqueza L, Valenzuela C, Delpón E, Longobardo M, Caballero R, Tamargo J . Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol 1998; 125: 969–78.
Paul AA, Witchel HJ, Hancox JC . Inhibition of the current of heterologously expressed hERG potassium channels by flecainide and comparison with quinidine, propafenone and lignocaine. Br J Pharmacol 2002; 136: 717–29.
Gao Z, Sun H, Chiu SW, Lau CP, Li GR . Effects of diltiazem and nifedipine on transient outward and ultra rapid delayed rectifier potassium currents in human atrial myocytes. Br J Pharmacol 2005; 144: 595–604.
Duan D, Fermini B, Nattel S . Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J Pharmacol Exp Ther 1993; 264: 1113–23.
Hoppe UC, Beuckelmann DJ . Modulation of the hyperpolarization activated inward current (If) by antiarrhythmic agents in isolated human atrial myocytes. Nauny Schmiedebergs Arch Pharmacol 1998; 358: 635–40.
Satoh H, Hashimoto K . Effect of propafenone on the membrane currents of rabbit sino-atrial node cells. Eur J Pharmacol 1984; 99: 185–91.
Delpon E, Valenzuela C, Perez O, Casis O, Tamargo J . Propafenone preferentially blocks the rapidly activating component of delayed rectifier K+ current in guinea pig ventricular myocytes. Voltage-independent and time-dependent block of the slowly activating component. Circ Res 1995; 76: 223–35.
Snyders J, Knoth KM, Roberds SL, Tamkun MM . Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol 1992; 41: 322–30.
Slawsky MT, Castle NA . K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. J Pharmacol Exp Ther 1994; 269: 66–74.
Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL . Modulation of hERG affinity for E-4031 by [K+]o and C-type inactivation. FEBS Lett 1997; 417: 43–47.
Nerbonne JM, Nichols CG, Schwarz TL, Escande D . Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here? Circ Res 2001; 89: 944–56.
Strauss HC, Morales MJ, Wang S, Brahmajothi MV, Campbell DL . Voltage-dependent K+ channels. In: Sperelakis N, Kurachi Y, Terzic A, Cohen MV editors; Heart physiology and pathophysiology. San Diego (California): Academic Press; 2001. p 259–80.
Acknowledgements
We thank Dr RASMUSSON (University at Buffalo, SUNY) for the fKv1.4ΔN cDNA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Wang, Sm., Chen, H. et al. Effects of diltiazem and propafenone on the inactivation and recovery kinetics of fKv1.4 channel currents expressed in Xenopus oocytes. Acta Pharmacol Sin 32, 465–477 (2011). https://doi.org/10.1038/aps.2010.234
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.234
Keywords
This article is cited by
-
Comparison of the effects of antiarrhythmic drugs flecainide and verapamil on fKv1.4ΔN channel currents in Xenopus oocytes
Acta Pharmacologica Sinica (2013)