Abstract
Aim:
To investigate the pharmacodynamics and pharmacokinetics of gemcitabine (dFdC) administered on d 1 and 5 plus cisplatin administered on d 1 in chemonaive patients with stage IIIB or IV non-small cell lung cancer (NSCLC).
Methods:
In each combination cycle, gemcitabine was administered at a dose of 1250 mg/m2 as a 30 min intravenous (iv) infusion on d 1 and 5 followed by cisplatin at a dose of 75 mg/m2 as a 3 h iv infusion on d 1 every 3 weeks. There was an interval of 1 h between the two infusions. Clinical response and toxicity of the regimen were observed. Furthermore, the plasma concentrations of gemcitabine (dFdC) and its metabolite (dFdU) at different time points were detected during the first cycle of infusion. Pharmacokinetic software (PKS) was used to estimate the pharmacokinetic parameters of gemcitabine and its metabolite dFdU.
Results:
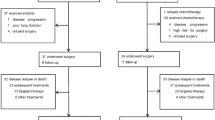
A total of 28 patients was enrolled in the study. The median age was 54 years (range 27–75 years), and most patients were in good clinical condition. Twenty-seven patients received two or more treatment cycles. The overall clinical response rate was 33.3%. The median overall survival time was 13 months. The estimated median time to tumor progression (TTP) was 6.2 months, and the 1-year survival rate was 55.6%. Toxicities were tolerated. The main toxicity was myelosuppression; 35.7% of patients had grade 3/4 hematologic toxicities and 28.6% had grade 3/4 non-hematologic toxicities, which were commonly gastrointestinal responses. The pharmacokinetic parameters of dFdC and dFdU were not different between pre- and post-administration of gemcitabine on d 1 and 5. dFdU was minimal (0.729±0.637 μg/mL) before gemcitabine was infused on d 5, and gemcitabine was not present.
Conclusion:
The regimen is active and well tolerated in chemonaive patients with advanced NSCLC. After gemcitabine was administered on d 1 and 5, the pharmacokinetic parameters of dFdC and dFdU showed no difference from those before the infusion, and dFdU was minimal before gemcitabine was administered on d 5.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ . Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 1996; 2: 521–30.
Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ . Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol 1995; 22 (4 Suppl 11): 72–9.
Belani CP . Chemotherapy regimens in advanced non-small-cell lung cancer: recent randomized trials. Clin Lung Cancer 2000; 2: S7–S10.
Le Chevalier T, Scagliotti G, Natale R, Danson S, Rosell R, Stahel R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer 2005; 47: 69–80.
Rossi D, Graziano F, Catalano V, Giordani P, Fedeli SL, Alessandroni P, et al. A new cisplatin/gemcitabine schedule in locally advanced (IIIB) and metastatic (IV) non-small cell lung cancer: relationship between dose-intensity and efficacy. A phase II study. Anticancer Res 2002; 22: 3087–92.
Jassem J, Krzakowski M, Roszkowski K, Ramlau R, Słomiński JM, Szczesna A, et al. A phase II study of gemcitabine plus cisplatin in patients with advanced non-small cell lung cancer: clinical outcomes and quality of life. Lung Cancer 2002; 35: 73–9.
van Zandwijk N, Smit EF, Kramer GW, Schramel F, Gans S, Festen J, et al. Gemcitabine and cisplatin as induction regimen for patients with biopsy-proven stage IIIA N2 non-small-cell lung cancer: a phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group (EORTC 08955). J Clin Oncol 2000; 18: 2658–64.
Shepherd FA, Cormier Y, Burkes R, Evans WK, Goss G, Klimo P, et al. Phase II trial of gemcitabine and weekly cisplatin for advanced non-small cell lung cancer. Semin Oncol 1997; 24: S8-27–S8-30.
Akcali Z, Calikusu Z, Sakalli H, Ozyilkan O . Gemcitabine and cisplatin treatment of advanced-stage non-small-cell lung cancer in patients given cisplatin on day 8. Tumori 2008; 94: 474–80.
Aydiner A, Kiyik M, Cikrikcioglu S, Kosar F, Gurses A, Turna A, et al. Gemcitabine and cisplatin as neo-adjuvant chemotherapy for non-small cell lung cancer: a phase II study. Lung Cancer 2007; 58: 246–52.
Aydiner A, Kiyik M, Cikrikcioglu S, Kosar F, Gurses A, Turna A, et al. A phase II study with gemcitabine and split-dose cisplatin in patients with advanced non-small cell lung cancer. Lung Cancer 2006; 54: 57–62.
Parra HS, Cavina R, Latteri F, Campagnoli E, Morenghi E, Torri W, et al. Cisplatin plus gemcitabine on days 1 and 4 every 21 days for solid tumors: result of a dose-intensity study. Invest New Drugs 2006; 25: 57–62.
Zwitter M, Kovac V, Smrdel U, Kocijancic I, Segedin B, Vrankar M . Phase I–II trial of low-dose gemcitabine in prolonged infusion and cisplatin for advanced non-small cell lung cancer. Anticancer Drugs 2005; 16: 1129–34.
López-Vivanco G, Viteri A, Barceló R, Muñoz A, Rubio I, Mañé JM, et al. Biweekly administration of cisplatin/gemcitabine in advanced non small cell lung cancer. Am J Clin Oncol 2005; 28: 501–7.
de Lange SM, van der Born K, Kroep JR, Jensen HA, Pfeiffer P, Cleverly A, et al. No evidence of gemcitabine accumulation during weekly administration. Eur J Clin Pharmacol 2005; 61: 843–9.
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 1991; 9: 491–8.
Lin NM, Zeng S, Ma SL, Fan Y, Zhong HJ, Fang L . Determination of gemcitabine and its metabolite in human plasma using high-pressure liquid chromatography coupled with a diode array detector. Acta Pharmacol Sin 2004; 25: 1584–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16.
Lin NM, Zeng S, Ma SL, Fan Y, Zhong HJ, Fang L . Pharmacokinetics study of gemcitabine and its metabolite in Chinese patients with malignant tumor. Chin Pharm J 2005; 40: 1089–92.
Hui YF, Reitz J . Gemcitabine: a cytidine analogue active against solid tumors. Am J Health Syst Pharm 1997; 54: 162–70.
Storniolo AM, Allerheiligen SR, Pearce HL . Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol 1997; 24: S7-2–S7-7.
Stephan AV, Dick P, Maria AJ, Bolijn MJ, Ong FH, Govindarajan R, et al. New insights into the pharmacology and cytotoxicity of gemcitabine and 2,2-difluorodeoxyuridine. Mol cancer Ther 2008; 7: 2415–25.
Veltkamp SA, Pluim D, van Tellingen O, Beijnen JH, Schellens JH . Extensive metabolism and hepatic accumulation of gemcitabine after multiple oral and intravenous administration in mice. Drug Metab Dispo 2008; 36: 1606–15.
Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol 2002; 20: 1657–67.
Gietema JA, Hoekstra R, de Vos FY, Uges DR, van der Gaast A, Groen HJ, et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann Oncol 2006; 17: 1320–7.
Acknowledgements
This study was financially supported by a grant from the Medical Science Research Foundation of Zhejiang Province, China (No 2008B027). We would like to thank Eli Lilly Company for providing a sample of dFdC.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fan, Y., Lin, Nm., Ma, Sl. et al. Phase II trial of gemcitabine plus cisplatin in patients with advanced non-small cell lung cancer. Acta Pharmacol Sin 31, 746–752 (2010). https://doi.org/10.1038/aps.2010.50
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.50
Keywords
This article is cited by
-
Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma
European Archives of Oto-Rhino-Laryngology (2012)