Abstract
Aim:
To develop a novel vehicle based on cubosomes as an ophthalmic drug delivery system for flurbiprofen (FB) to reduce ocular irritancy and improve bioavailability.
Methods:
FB-loaded cubosomes were prepared using hot and high-pressure homogenization. Cubosomes were then characterized by particle size, zeta potential, encapsulation efficiency, particle morphology, inner cubic structure and in vitro release. Corneal permeation was evaluated using modified Franz-type cells. Ocular irritation was then evaluated using both the Draize method and histological examination. The ocular pharmacokinetics of FB was determined using microdialysis.
Results:
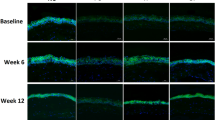
The particle size of each cubosome formulation was about 150 nm. A bicontinuous cubic phase of cubic P-type was determined using cryo-transmission electron microscopy (cryo-TEM) observation and small angle X-ray scattering (SAXS) analysis. In vitro corneal permeation study revealed that FB formulated in cubosomes exhibited 2.5-fold (F1) and 2.0-fold (F2) increase in Papp compared with FB PBS. In the ocular irritation test, irritation scores for each group were less than 2, indicating that all formulations exhibited excellent ocular tolerance. Histological examination revealed that neither the structure nor the integrity of the cornea was visibly affected after incubation with FB cubosomes. The AUC of FB administered as FB cubosome F2 was 486.36±38.93 ng·mL−1·min·μg−1, which was significantly higher than that of FB Na eye drops (P<0.01). Compared with FB Na eye drops, the Tmax of FB cubosome F2 was about 1.6-fold higher and the MRT was also significantly longer (P<0.001).
Conclusion:
This novel low-irritant vehicle based on cubosomes might be a promising system for effective ocular drug delivery.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Waterbury L, Kunysz EA, Bewerman R . Effect of steroidal and non steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol 1987; 3: 43–54.
Schalnus R . Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica 2003; 217: 89–98.
Joseph TM . A critical look at ocular allergy drugs. Am Fam Physician 1996; 53: 2637–42.
Stroobants A, Fabre K, Maudgal PC . Effect of non-steroidal antiinflammatory drugs (NSAID) on the rabbit corneal epithelium studied by scanning electron microscopy. Bull Soc Belge Ophtalmol 2000; 276: 73–81.
Araújo J, Vega E, Lopes C, Egea MA, Garcia ML, Souto EB . Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloid Surfaces B 2009; 72: 48–56.
Label information of Ocufen. Label approved on 08/25/2003. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19404scp020_ocufen_lbl.pdf
Ahuja M, Dhake AS, Sharma SK, Majumdar DK . Topical ocular delivery of NSAIDs. AAPS J 2008; 10: 229–41.
Li N, Zhuang CY, Wang M, Sun XY, Nie SF, Pan WS . Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm 2009; 379: 131–8.
Gan L, Gan Y, Zhu CL, Zhang XX, Zhu JB . Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm 2009; 365: 143–9.
Ibrahim HK, El-Leithy IS, Makky AA . Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol Pharm 2010; 7: 576–85.
Nakano M, Sugita A, Matsuoka H, Handa T . Small-angle X-ray scattering and 13C-NMR investigation on the internal structure of “cubosomes”. Langmuir 2001; 17: 3917–22.
Malmsten M . Soft drug delivery systems. Soft Matter 2006; 2: 760–9.
Zhao XY, Zhang J, Zheng LQ, Li DH . Studies of cubosomes as a sustained drug delivery system. J Disp Sci Tech 2004; 25: 795–9.
Larsson K . Aqueous dispersion of cubic lipid water phases. Curr Opin Colloid Interface Sci 2000; 5: 64–9.
Garg G, Saraf S, Saraf S . Cubosomes: an overview. Biol Pharm Bull 2007; 30: 350–3.
Johnsson M, Barauskas J, Norlin A, Tiberg F . Physicochemical and drug delivery aspects of lipid-based liquid crystalline nanoparticles: a case study of intravenously administered propofol. J Nanosci Nanotechnol 2006; 6: 3017–24.
Chung H, Kim J, Um JY, Kwon IC, Jeong SY . Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia 2002; 45: 448–51.
Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res 2005; 22: 2163–73.
Larsson K . Cubic lipid-water phases: Structures and biomembrane aspects. J Phys Chem 1989; 93: 7304–14.
Gupta PK, Hung CT, Perrier DG . Quantitation of the release of doxorubicin from colloidal dosage forms using dynamic dialysis. J Pharm Sci 1987; 76: 141–5.
Rozier A, Manuel C, Groove J, Plazonet B . Gelrite: a novel ion activated, in situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm 1989; 57: 163–8.
O'Brien WJ, Edelhauser HF . The corneal penetration of trifluorothymidine, adenine -arabinoside, and idoxuidine: a comparative study. Invest Ophthalmol Vis Sci 1977; 16: 1093–103.
Baydoun L, Furrer P, Gurny R, Muller-Goymann CC . New surface-active polymers for ophthalmic formulations: evaluation of ocular tolerance. Eur J Pharm Biopharm 2004; 58: 169–75.
Tak RV, Pal D, Gao H, Dey S, Mitra AK . Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J Pharm Sci 2001; 90: 1505–15.
Neto C, Aloisi G, Baglioni P, Larsson K . Imaging soft-matter with the atomic force microscopy: cubosomes and hexosomes. J Phys Chem B 1999; 103: 3896–9.
Spicer PT . Progress in liquid crystalline dispersions: cubosomes. Curr Opin Colloid Interf Sci 2005; 10: 274–9.
Boyd BJ . Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm 2003; 260: 239–47.
Saettone MF, Chetoni P, Cerbai R, Mazzanti G, Braghiroli L . Evaluation of ocular permeation enhancers: In vitro effects on corneal transport of four [beta]-blockers and in vitro/in vivo toxic activity. Int J Pharm 1996; 142: 103–13.
Rittenhouse KD, Peiffer RL, Pollack GM . Evaluation of microdialysis sampling of aqueous humor for in vivo models of ocular absorption and disposition. J Pharmaceut Biomed 1998; 16: 951–9.
Katragadda S, Gunda S, Hariharan S, Mitra AK . Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: Evaluation of their utility in treating ocular HSV infections. Int J Pharm 2008; 359: 15–24.
Levy MY, Schutze W, Fuhrer C, Benita S . Characterization of diazepam submicron emulsion interface: role of oleic acid. J Microencapsul 1994; 11: 79–92.
Lopes LB, Speretta FFF, Bentley MVLB . Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur J Pharmaceut Sci 2007; 32: 209–15.
Acknowledgements
The authors are grateful to Danisco Cultor, Denmark, for providing samples of GMO. We thank National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (No 2009ZX09301-001) for the financial support. This work was also supported in part by the National Basic Research Program of China (No 2009CB930300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, S., Shen, Jq., Gan, Y. et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin 31, 990–998 (2010). https://doi.org/10.1038/aps.2010.98
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.98
Keywords
This article is cited by
-
Preclinical assessment of nanostructured liquid crystalline particles for the management of bacterial keratitis: in vivo and pharmacokinetics study
Drug Delivery and Translational Research (2022)
-
In vitro and in vivo evaluation of cubosomal nanoparticles as an ocular delivery system for fluconazole in treatment of keratomycosis
Drug Delivery and Translational Research (2020)
-
Atorvastatin-loaded solid lipid nanoparticles as eye drops: proposed treatment option for age-related macular degeneration (AMD)
Drug Delivery and Translational Research (2020)
-
Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis
Pharmaceutical Research (2020)
-
Lyotropic liquid crystal systems in drug delivery: a review
Journal of Pharmaceutical Investigation (2015)