Abstract
Aim:
To observe the effect of Rhein lysinate (RHL) on cellular senescence of human umbilical vascular endothelial cells (HUVECs) and elucidate its action mechanism.
Methods:
Cell viability was determined using MTT assay. The expression levels of Sirt1 mRNA and protein were measured by RT-PCR and Western blot, respectively. Senescence associated (SA)-β-galactosidase activity was detected to evaluate cell senescence. Apoptosis and cell cycle progression were determined using flow cytometry.
Results:
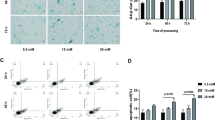
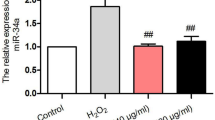
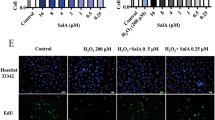
Treatment with RHL (10 μmol/L) for 48 h significantly increased the proliferation of HUVECs. In contrast, treatment with H2O2 (25, 50 and 100 μmol/L) for 6 d dose-dependently increased β-galactosidase positive cells. Spontaneous cell senescence appeared as the cell passage increased. Pre-treatment with RHL (10 μmol/L) reversed H2O2 or increased cell passage-induced cell senescence. H2O2 (100 μmol/L) significantly arrested HUVECs at G1 phase (73.8% vs 64.6% in the vehicle group), which was blocked by RHL (10 μmol/L). RHL (5 and 10 μmol/L) enhanced both mRNA transcription and protein expression of Sirt1. H2O2 (100 μmol/L) significantly decreased Sirt1 expression, and induced up-regulation of p53 acetylation and p16INK4a, which were blocked by pre-treatment with RHL (10 μmol/L). Interference with siRNA for Sirt1 abolished the effect of RHL. H2O2 (100 μmol/L) did not induce HUVEC apoptosis. The expression of apoptosis-associated proteins, such as p53, p21, Bcl-2, and Bax, did not significantly change in the presence of H2O2 (100 μmol/L) or RHL (10 μmol/L).
Conclusion:
RHL protected HUVECs against cellular senescence induced by H2O2, via up-regulation of Sirt1 expression and down-regulation of the expression of acetyl-p53 and p16INK4a.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brandes RP, Fleming I, Busse R . Endothelial aging. Cardiovasc Res 2005; 66: 286–94.
Muller G, Morawietz H . Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal 2009; 11: 1711–31.
Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 2006; 339: 827–32.
Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, et al. Sirt1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS One 2008; 3: e1710.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress dependent regulation of FOXO transcription factors by the Sirt1 deacetylase. Science 2004; 303: 2011–5.
Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, et al. Human Sir2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 2002; 21: 2383–96.
Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, et al. Interactions between E2F1 and Sirt1 regulate apoptotic response to DNA damage. Nat Cell Biol 2006; 8: 1025–31.
Huang Q, Lu G, Shen HM, Chung MC, Ong CN . Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev 2007; 27: 609–30.
Martin G, Bogdanowicz P, Domagala F, Ficheux H, Pujol JP . Articular chondrocytes cultured in hypoxia: their response to interleukin-1beta and rhein, the active metabolite of diacerhein. Biorheology 2004; 41: 549–61.
Tan ZH, Shen YJ, Zhao JN, Li HY, Zhang J . Effects of rhein on the function of human mesangial cells in high glucose environment. Yao Xue Xue Bao 2004; 39: 881–6.
Lin ML, Chen SS, Lu YC, Liang RY, Ho YT, Yang CY, et al. Rhein induces apoptosis through induction of endoplasmic reticulum stress and Ca2+-dependent mitochondrial death pathway in human nasopharyngeal carcinoma cells. Anticancer Res 2007; 27: 3313–22.
Lin YJ, Zhen YS . Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of Taxol in athymic mice. Anticancer Drugs 2009; 20: 65–72.
Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, et al. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 2008; 28: 1634–9.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92: 9363–7.
Fang JH, Wang XH, Xu ZR, Jiang FG . Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci 2010; 11; 31.
Gruber HE, Ingram JA, Davis DE, Hanley EN Jr . Increased cellular senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J 2009; 9: 210–5.
Ben-Porath I, Weinberg RA . The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 2005; 37: 961–76.
Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W . Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A 2004; 101: 2259–64.
Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S . H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of Sirt1 by NAD+ depletion. Cell Physiol Biochem 2007; 20: 45–54.
Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, et al. Sirt1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res 2010; 106: 1384–93.
Cai L, Wang H, Li Q, Qian Y, Yao W . Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim Biophys Sin 2008; 40: 796–802.
Dai CY, Enders GH . p16 INK4a can initiate an autonomous senescence program. Oncogene 2000; 19: 1613–22.
Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005; 436: 720–4.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No 81001439).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Yj., Zhen, Yz., Wei, J. et al. Effects of Rhein Lysinate on H2O2-induced cellular senescence of human umbilical vascular endothelial cells. Acta Pharmacol Sin 32, 1246–1252 (2011). https://doi.org/10.1038/aps.2011.101
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2011.101
Keywords
This article is cited by
-
Rhein attenuates angiotensin II-induced cardiac remodeling by modulating AMPK–FGF23 signaling
Journal of Translational Medicine (2022)
-
Mechanistic perspectives of calorie restriction on vascular homeostasis
Science China Life Sciences (2014)
-
Rhein lysinate increases the median survival time of SAMP10 mice: protective role in the kidney
Acta Pharmacologica Sinica (2013)
-
Rhein lysinate inhibits monocyte adhesion to human umbilical vein endothelial cells by blocking p38 signaling pathway
Archives of Pharmacal Research (2013)