Abstract
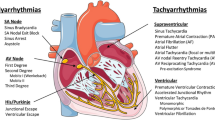
Antiarrhythmic drugs are a group of pharmaceuticals that suppress or prevent abnormal heart rhythms, which are often associated with substantial morbidity and mortality. Current antiarrhythmic drugs that typically target plasma membrane ion channels have limited clinical success and in some cases have been described as being pro-arrhythmic. However, recent studies suggest that pathological release of calcium (Ca2+) from the sarcoplasmic reticulum via cardiac ryanodine receptors (RyR2) could represent a promising target for antiarrhythmic therapy. Diastolic SR Ca2+ release has been linked to arrhythmogenesis in both the inherited arrhythmia syndrome 'catecholaminergic polymorphic ventricular tachycardia' and acquired forms of heart disease (eg, atrial fibrillation, heart failure). Several classes of pharmaceuticals have been shown to reduce abnormal RyR2 activity and may confer protection against triggered arrhythmias through reduction of SR Ca2+ leak. In this review, we will evaluate the current pharmacological methods for stabilizing RyR2 and suggest treatment modalities based on current evidence of molecular mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bers DM . Cardiac excitation-contraction coupling. Nature 2002; 415: 198–205.
Wehrens XH, Lehnart SE, Marks AR . Intracellular calcium release and cardiac disease. Annu Rev Physiol 2005; 67: 69–98.
Dobrev D, Wehrens XH . Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med 2010; 20: 30–4.
Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001; 103: 485–90.
Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 2003; 113: 829–40.
Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 2009; 119: 1940–51.
Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005; 111: 2025–32.
Mathur N, Sood S, Wang S, van Oort RJ, Sarma S, Li N, et al. Sudden infant death syndrome in mice with an inherited mutation in RyR2. Circ Arrhythm Electrophysiol 2009; 2: 677–85.
Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001; 103: 196–200.
van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation 2010; 122: 2669–79.
Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A 2006; 103: 7906–10.
Paavola J, Viitasalo M, Laitinen-Forsblom PJ, Pasternack M, Swan H, Tikkanen I, et al. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur Heart J 2007; 28: 1135–42.
McCauley MD, Wehrens XH . Animal models of arrhythmogenic cardiomyopathy. Dis Model Mech 2009; 2: 563–70.
Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol 2007; 42: 1026–35.
Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 2007; 117: 1814–23.
Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res 2009; 84: 387–95.
Gonzalez DR, Beigi F, Treuer AV, Hare JM . Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A 2007; 104: 20612–7.
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000; 101: 365–76.
Wehrens XH, Lehnart SE, Reiken SR, Marks AR . Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 2004; 94: e61–70.
Xu L, Eu JP, Meissner G, Stamler JS . Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 1998; 279: 234–7.
Anderson ME, Higgins LS, Schulman H . Disease mechanisms and emerging therapies: protein kinases and their inhibitors in myocardial disease. Nat Clin Pract Cardiovasc Med 2006; 3: 437–45.
Santonastasi M, Wehrens XH . Ryanodine receptors as pharmacological targets for heart disease. Acta Pharmacol Sin 2007; 28: 937–44.
Dulhunty AF, Beard NA, Pouliquin P, Casarotto MG . Agonists and antagonists of the cardiac ryanodine receptor: potential therapeutic agents? Pharmacol Ther 2007; 113: 247–63.
Wehrens XH, Lehnart SE, Marks AR . Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci 2005; 1047: 366–75.
Snyder HR Jr, Davis CS, Bickerton RK, Halliday RP . 1-[(5-arylfurfurylidene)amino]hydantoins. A new class of muscle relaxants. J Med Chem 1967; 10: 807–10.
Harrison GG . Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br J Anaesth 1975; 47: 62–5.
Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008; 133: 53–65.
Denborough M . Malignant hyperthermia. Lancet 1998; 352: 1131–6.
Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, et al. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem 2002; 277: 34918–23.
Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N . Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem 2005; 280: 6580–7.
Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol 2009; 53: 1993–2005.
Szentesi P, Collet C, Sarkozi S, Szegedi C, Jona I, Jacquemond V, et al. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol 2001; 118: 355–75.
Balam Ortiz EO, Carvajal K, Cruz D . Protective effect of dantrolene in post-ischemic reperfusion myocardial damage. Arch Inst Cardiol Mex 1999; 69: 311–9.
Boys JA, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH . Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart, brain, liver, and kidney. J Investig Med 2010; 58: 875–82.
Pelleg A, Roth A, Shargordsky B, Belhassen B, Chagnac A, Laniado S . Effects of dantrolene sodium on occlusion and reperfusion arrhythmias in the canine heart. Methods Find Exp Clin Pharmacol 1985; 7: 239–43.
Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J 2010; 74: 2579–84.
Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun 2010; 394: 660–6.
Meissner A, Min JY, Haake N, Hirt S, Simon R . Dantrolene sodium improves the force-frequency relationship and beta-adregenic res-ponsiveness in failing human myocardium. Eur J Heart Fail 1999; 1: 177–86.
Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, et al. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res 2010; 87: 609–17.
Kaneko N . New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res 1994; 33: 429–38.
Kumagai K, Nakashima H, Gondo N, Saku K . Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol 2003; 14: 880–4.
Hachida M, Lu H, Kaneko N, Nonoyama M, Koyanagi H . Protective effect of JTV519 on prolonged myocardial preservation. Transplant Proc 1999; 31: 1094.
Inagaki K, Kihara Y, Hayashida W, Izumi T, Iwanaga Y, Yoneda T, et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of delta-isoform of protein kinase C in rat ventricular myocardium. Circulation 2000; 101: 797–804.
Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H . Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol 1999; 79: 275–81.
Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemura H . Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br J Pharmacol 2000; 131: 1363–72.
Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 2003; 107: 477–84.
Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 2004; 304: 292–6.
Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol Heart Circ Physiol 2003; 284: H1035–42.
Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 2004; 109: 3208–14.
Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J 2007; 404: 431–8.
Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res 2006; 99: 292–8.
Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, et al. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res 2010; 87: 50–9.
Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, et al. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation 2008; 117: 762–72.
Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 2008; 118: 2230–45.
Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A 2008; 105: 2198–202.
Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 2010; 107: 1559–64.
Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest 2010; 120: 4375–87.
Banitt EH, Bronn WR, Coyne WE, Schmid JR . Antiarrhythmics. 2. Synthesis and antiarrhythmic activity of N-(piperidylalkyl)trifluoroethoxybenzamides. J Med Chem 1977; 20: 821–6.
Anderson JL, Stewart JR, Perry BA, Van Hamersveld DD, Johnson TA, Conard GJ, et al. Oral flecainide acetate for the treatment of ventricular arrhythmias. N Engl J Med 1981; 305: 473–7.
Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, Bhandari AK, et al. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation 1989; 80: 1557–70.
Muhiddin K, Nathan AW, Hellestrand KJ, Banim SO, Camm AJ . Ventricular tachycardia associated with flecainide. Lancet 1982; 2: 1220–1.
Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321: 406–12.
Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med 2009; 15: 380–3.
Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol 2010; 48: 293–301.
Kang G, Giovannone SF, Liu N, Liu FY, Zhang J, Priori SG, et al. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res 2010; 107: 512–9.
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 2009; 15: 325–30.
Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM . Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 2010; 285: 28938–45.
Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E . Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol 2009; 297: H1992–2003.
Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008; 133: 462–74.
Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail 2009; 2: 664–75.
Erickson JR, Anderson ME . CaMKII and its role in cardiac arrhythmia. J Cardiovasc Electrophysiol 2008; 19: 1332–6.
Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 2005; 123: 25–35.
Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 2003; 107: 2459–66.
Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362: 7–13.
Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR . Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A 2006; 103: 511–8.
Acknowledgements
Dr McCauley is supported by NHLBI training grant 5T32HL066991-07. Dr WEHRENS is a WM Keck Foundation Distinguished Young Scholar in Medical Research and is supported by NHLBI grants R01-HL089598 and R01-HL091947, and Muscular Dystrophy Association grant 186531. This work was also supported in part by the Fondation Leducq Alliance for CaMKII Signaling in Heart.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCauley, M., Wehrens, X. Targeting ryanodine receptors for anti-arrhythmic therapy. Acta Pharmacol Sin 32, 749–757 (2011). https://doi.org/10.1038/aps.2011.44
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2011.44
Keywords
This article is cited by
-
p38γ/δ activation alters cardiac electrical activity and predisposes to ventricular arrhythmia
Nature Cardiovascular Research (2023)
-
SOCE in the cardiomyocyte: the secret is in the chambers
Pflügers Archiv - European Journal of Physiology (2021)
-
RyR1-targeted drug discovery pipeline integrating FRET-based high-throughput screening and human myofiber dynamic Ca2+ assays
Scientific Reports (2020)
-
Altered sarcoplasmic reticulum calcium cycling—targets for heart failure therapy
Nature Reviews Cardiology (2012)
-
Channelopathies and drug discovery in the postgenomic era
Acta Pharmacologica Sinica (2011)