Abstract
Aim:
To investigate how co-delivery of the gene encoding C–C chemokine ligand-19 (CCL-19) affected the systemic immune responses to an anti-caries DNA vaccine pCIA-P in mice.
Methods:
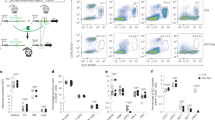
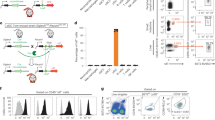
Plasmid encoding CCL19-GFP fusion protein (pCCL19/GFP) was constructed by inserting murine ccl19 gene into GFP-expressing vector pAcGFP1-N1. Chemotactic effect of the fusion protein on murine dendritic cells (DCs) was assessed in vitro and in vivo using transwell and flow cytometric analysis, respectively. BALB/c mice were administered anti-caries DNA vaccine pCIA-P plus pCCL19/GFP (each 100 μg, im) or pCIA-P alone. Serum level of anti-PAc IgG was assessed with ELISA. Splenocytes from the mice were stimulated with PAc protein for 48 h, and IFN-γ and IL-4 production was measured with ELISA. The presence of pCCL19/GFP in spleen and draining lymph nodes was assessed using PCR. The expression of pCCL19/GFP protein in these tissues was analyzed under microscope and with flow cytometry.
Results:
The expression level of CCL19-GFP fusion protein was considerably increased 48 h after transfection of COS-7 cells with pCCL19/GFP plasmids. The fusion protein showed potent chemotactic activity on DCs in vitro. The level of serum PAc-specific IgG was significantly increased from 4 to 14 weeks in the mice vaccinated with pCIA-P plus pCCL19/GFP. Compared to mice vaccinated with pCIA-P alone, the splenocytes from mice vaccinated with pCIA-P plus pCCL19/GFP produced significantly higher level of IFN-γ, but IL-4 production had no significant change. Following intromuscular co-delivery, pCCL19/GFP plasmid and fusion protein were detected in the spleen and draining lymph nodes. Administration of CCL19 gene in mice markedly increased the number of mature DCs in secondary lymphoid tissues.
Conclusion:
CCL19 serves as an effective adjuvant for anti-caries DNA vaccine by inducing chemotactic migration of DCs to secondary lymphoid tissues.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Donnelly JJ, Wahren B, Liu MA . DNA vaccines: progress and challenges. J Immunol 2005; 175: 633–9.
Kutzler MA, Weiner DB . DNA vaccines: ready for prime time? Nat Rev Genet 2008; 9: 776–88.
Fan MW, Bian Z, Peng ZX, Zhong Y, Chen Z, Peng B, et al. A DNA vaccine encoding a cell-surface protein antigen of Streptococcus mutans protects gnotobiotic rats from caries. J Dent Res 2002; 81: 784–7.
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247: 1465–8.
Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN . Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med 1998; 188: 1075–82.
Bot A, Stan AC, Inaba K, Steinman R, Bona C . Dendritic cells at a DNA vaccination site express the encoded influenza nucleoprotein and prime MHC class I-restricted cytolytic lymphocytes upon adoptive transfer. Int Immunol 2000; 12: 825–32.
Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B . DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med 1999; 189: 169–78.
Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD Jr. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med 1996; 2: 1122–8.
Mitoma M, Oho T, Michibata N, Okano K, Nakano Y, Fukuyama M, et al. Passive immunization with bovine milk containing antibodies to a cell surface protein antigen-glucosyltransferase fusion protein protects rats against dental caries. Infect Immun 2002; 70: 2721–4.
Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 1998; 188: 373–86.
Sallusto F, Lanzavecchia A . Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med 1999; 189: 611–4.
Scheerlinck JY . Genetic adjuvants for DNA vaccines. Vaccine 2001; 19: 2647–56.
Zlotnik A, Yoshie O . Chemokines: a new classification system and their role in immunity. Immunity 2000; 12: 121–7.
Campbell JJ, Butcher EC . Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 2000; 12: 336–41.
Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33.
Cyster JG . Chemokines and cell migration in secondary lymphoid organs. Science 1999; 286: 2098–102.
Zlotnik A, Morales J, Hedrick JA . Recent advances in chemokines and chemokine receptors. Crit Rev Immunol 1999; 19: 1–47.
Sallusto F, Lanzavecchia A . Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev 2000; 177: 134–40.
Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG . Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A 2000; 97: 12694–9.
Eo SK, Lee S, Kumaraguru U, Rouse BT . Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine 2001; 19: 4685–93.
Lee Y, Eo SK, Rouse RJ, Rouse BT . Influence of CCR7 ligand DNA preexposure on the magnitude and duration of immunity. Virology 2003; 312: 169–80.
Toka FN, Gierynska M, Rouse BT . Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J Virol 2003; 77: 12742–52.
Han YW, Aleyas AG, George JA, Kim SJ, Kim HK, Yoo DJ, et al. Genetic co-transfer of CCR7 ligands enhances immunity and prolongs survival against virulent challenge of pseudorabies virus. Immunol Cell Biol 2009; 87: 91–9.
Westermann J, Nguyen-Hoai T, Baldenhofer G, Höpken UE, Lipp M, Dörken B, et al. CCL19 (ELC) as an adjuvant for DNA vaccination: induction of a TH1-type T-cell response and enhancement of antitumor immunity. Cancer Gene Ther 2007; 14: 523–32.
Nguyen-Hoai T, Baldenhofer G, Ahmed MS, Pham-Duc M, Gries M, Lipp M, et al. CCL19 (ELC) improves TH1-polarized immune responses and protective immunity in a murine Her2/neu DNA vaccination model. J Gene Med 2012; 14: 128–37.
Xu Q, Katz J, Zhang P, Ashtekar AR, Gaddis DE, Fan M, et al. Contribution of a Streptococcus mutans antigen expressed by a Salmonella vector vaccine in dendritic cell activation. Infect Immun 2011; 79: 3792–800.
Xu QA, Yu F, Fan MW, Bian Z, Chen Z, Fan B, et al. Immunogenicity and persistence of a targeted anti-caries DNA vaccine. J Dent Res 2006; 85: 915–8.
Kutzler MA, Weiner DB . Developing DNA vaccines that call to dendritic cells. J Clin Invest 2004; 114: 1241–4.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998; 392: 245–52.
Mellman I, Steinman RM . Dendritic cells: specialized and regulated antigen processing machines. Cell 2001; 106: 255–8.
Wen H, Schaller MA, Dou Y, Hogaboam CM, Kunkel SL . Dendritic cells at the interface of innate and acquired immunity: the role for epigenetic changes. J Leukoc Biol 2008; 83: 439–46.
Tuomela M, Malm M, Wallen M, Stanescu I, Krohn K, Peterson P . Biodistribution and general safety of a naked DNA plasmid, GTU-MultiHIV, in a rat, using a quantitative PCR method. Vaccine 2005; 23: 890–6.
Coelho-Castelo AA, Trombone AP, Rosada RS, Santos RR Jr, Bonato VL, Sartori A, et al. Tissue distribution of a plasmid DNA encoding Hsp65 gene is dependent on the dose administered through intramuscular delivery. Genet Vaccines Ther 2006; 4: 1.
Liu C, Fan M, Xu Q, Li Y . Biodistribution and expression of targeted fusion anti-caries DNA vaccine pGJA-P/VAX in mice. J Gene Med 2008; 10: 298–305.
Iwasaki A, Torres CAT, Ohashi PS, Robinson HL, Barber BH . The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol 1997; 159: 11–4.
Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem 1997; 272: 13803–9.
Sánchez-Sánchez N, Riol-Blanco L, Rodríguez-Fernández JL . The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol 2006; 176: 5153–9.
Liu GX, Xu QA, Jin J, Li YH, Jia R, Guo JH, et al. Mucosal and systemic immunization with targeted fusion anti-caries DNA plasmid in young rats. Vaccine 2009; 27: 2940–7.
Li YH, Huang S, Du M, Bian Z, Chen Z, Fan MW . Immunogenic characterization and protection against Streptococcus mutans infection induced by intranasal DNA prime-protein boost immunization. Vaccine 2010; 28: 5370–6.
Pertmer TM, Roberts TR, Haynes JR . Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol 1996; 70: 6119–25.
Feltquate DM, Heaney S, Webster RG, Robinson HL . Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol 1997; 158: 2278–84.
Navia JM . Animalmodels in dental research. Tuscaloosa: University of Alabama Press; 1977. p 280.
Wang S, Heilman D, Liu F, Giehl T, Joshi S, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 2004; 22: 3348–57.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No 81000435), the Doctoral Fund of the Ministry of Education of China (No 200804861011), and the PhD Candidates Self-research (including 1+4) Program of Wuhan University in 2010 (No 2010304010100163).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, Yh., Qi, Sc., Su, Lk. et al. Co-delivery of ccl19 gene enhances anti-caries DNA vaccine pCIA-P immunogenicity in mice by increasing dendritic cell migration to secondary lymphoid tissues. Acta Pharmacol Sin 34, 432–440 (2013). https://doi.org/10.1038/aps.2012.153
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.153
Keywords
This article is cited by
-
CCL17 combined with CCL19 as a nasal adjuvant enhances the immunogenicity of an anti-caries DNA vaccine in rodents
Acta Pharmacologica Sinica (2016)
-
CCL4 as an adjuvant for DNA vaccination in a Her2/neu mouse tumor model
Cancer Gene Therapy (2016)