Abstract
Aim:
To investigate the effects of the potassium-sparing diuretic amiloride on endothelial cell apoptosis during lipopolysaccharide (LPS)-accelerated atherosclerosis.
Methods:
Human umbilical vein endothelial cells (HUVECs) were exposed to LPS (100 ng/mL) in the presence of drugs tested. The activity of Na+/H+ exchanger 1 (NHE1) and calpain, intracellular free Ca2+level ([Ca2+]i), as well as the expression of apoptosis-related proteins in the cells were measured. For in vivo study, ApoE-deficient (ApoE−/−) mice were fed high-fat diets with 0.5% (w/w) amiloride for 4 weeks and LPS (10 μg/mouse) infusion into caudal veins. Afterwards, atherosclerotic lesions, NHE1 activity and Bcl-2 expression in the aortic tissues were evaluated.
Results:
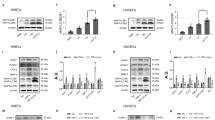
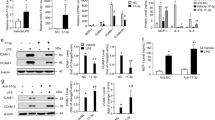
LPS treatment increased NHE1 activity and [Ca2+]i in HUVECs in a time-dependent manner, which was associated with increased activity of the Ca2+-dependent protease calpain. Amiloride (1−10 μmol/L) significantly suppressed LPS-induced increases in NHE1 activity, [Ca2+]i. and calpain activity. In the presence of the Ca2+ chelator BAPTA (0.5 mmol/L), LPS-induced increase of calpain activity was also abolished. In LPS-treated HUVECs, the expression of Bcl-2 protein was significantly decreased without altering its mRNA level. In the presence of amiloride (10 μmol/L) or the calpain inhibitor ZLLal (50 μmol/L), the down-regulation of Bcl-2 protein by LPS was blocked. LPS treatment did not alter the expression of Bax and Bak proteins in HUVECs. In the presence of amiloride, BAPTA or ZLLal, LPS-induced HUVEC apoptosis was significantly attenuated. In ApoE−/− mice, administration of amiloride significantly suppressed LPS-accelerated atherosclerosis and LPS-induced increase of NHE1 activity, and reversed LPS-induced down-regulation of Bcl-2 expression.
Conclusion:
LPS stimulates NHE1 activity, increases [Ca2+]i, and activates calpain, which leads to endothelial cell apoptosis related to decreased Bcl-2 expression. Amiloride inhibits NHE1 activity, thus attenuates LPS-accelerated atherosclerosis in mice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Teiwes J, Toto RD . Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens 2007; 20: 109–17.
Raetz CR . Biochemistry of endotoxins. Annu Rev Biochem 1990; 59: 129–70.
Liu T, Huang Y, Likhotvorik RI, Keshvara L, Hoyt DG . Protein Never in Mitosis Gene A Interacting-1 (PIN1) regulates degradation of inducible nitric oxide synthase in endothelial cells. Am J Physiol Cell Physiol 2008; 295: C819–27.
Cybulsky MI, Chan MK, Movat HZ . Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest 1988; 58: 365–78.
Pober JS, Cotran RS . The role of endothelial cells in inflammation. Transplantation 1990; 50: 537–44.
Choi KB, Wong F, Harlan JM, Chaudhary PM, Hood L, Karsan A . Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem 1998; 273: 20185–8.
Wang SX, Xiong XM, Song T, Liu LY . Protective effects of cariporide on endothelial dysfunction induced by high glucose. Acta Pharmacol Sin 2005; 26: 329–33.
Wang D, Dou K, Song Z, Liu Z . The Na+/H+ exchange inhibitor: a new therapeutic approach for hepatic ischemia injury in rats. Transplant Proc 2003; 35: 3134–5.
Goll DE, Thompson VF, Li H, Wei W, Cong J . The calpain system. Physiol Rev 2003; 83: 731–801.
Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem 2002; 277: 27217–26.
Schelling JR, Abu Jawdeh BG . Regulation of cell survival by Na+/H+exchanger-1. Am J Physiol Renal Physiol 2008; 295: F625–32.
Garciarena CD, Caldiz CI, Portiansky EL, Chiappe de Cingolani GE, Ennis IL . Chronic NHE-1 blockade induces an antiapoptotic effect in the hypertrophied heart. J Appl Physiol 2009; 106: 1325–31.
Wang S, Xu J, Song P, Wu Y, Zhang J, Chul Choi H, et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension 2008; 52: 484–90.
Kyronlahti A, Ramo M, Tamminen M, Unkila-Kallio L, Butzow R, Leminen A, et al. GATA-4 regulates Bcl-2 expression in ovarian granulosa cell tumors. Endocrinology 2008; 149: 5635–42.
Wang SX, Sun XY, Zhang XH, Chen SX, Liu YH, Liu LY . Cariporide inhibits high glucose-mediated adhesion of monocyte-endothelial cell and expression of intercellular adhesion molecule-1. Life Sci 2006; 79: 1399–404.
Dong Y, Tan J, Cui MZ, Zhao G, Mao G, Singh N, et al. Calpain inhibitor MDL28170 modulates Abeta formation by inhibiting the formation of intermediate Abeta46 and protecting Abeta from degradation. Faseb J 2006; 20: 331–3.
Wang S, Peng Q, Zhang J, Liu L . Na+/H+ exchanger is required for hyperglycemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovasc Res 2008; 80: 255–62.
Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH . Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem 2008; 283: 12446–55.
Nakatani K, Takeshita S, Tsujimoto H, Sekine I . Intravenous immunoglobulin (IVIG) preparations induce apoptosis in TNF-alpha-stimulated endothelial cells via a mitochondria-dependent pathway. Clin Exp Immunol 2002; 127: 445–54.
Bannerman DD, Goldblum SE . Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol 2003; 284: L899–914.
Toda T, Kadono T, Hoshiai M, Eguchi Y, Nakazawa S, Nakazawa H, et al. Na+/H+ exchanger inhibitor cariporide attenuates the mitochondrial Ca2+ overload and PTP opening. Am J Physiol Heart Circ Physiol 2007; 293: H3517–23.
Koliakos G, Befani C, Paletas K, Kaloyianni M . Effect of endothelin on sodium/hydrogen exchanger activity of human monocytes and atherosclerosis-related functions. Ann N Y Acad Sci 2007; 1095: 274–91.
Azuma M, Shearer TR . The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol 2008; 53: 150–63.
Stalker TJ, Gong Y, Scalia R . The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes 2005; 54: 1132–40.
Stalker TJ, Skvarka CB, Scalia R . A novel role for calpains in the endothelial dysfunction of hyperglycemia. Faseb J 2003; 17: 1511–3.
Lessene G, Czabotar PE, Colman PM . BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 2008; 7: 989–1000.
Yip KW, Reed JC . Bcl-2 family proteins and cancer. Oncogene 2008; 27: 6398–406.
Fletcher JI, Huang DC . Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle 2008; 7: 39–44.
Chen K, Keaney J . Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium 2004; 11: 109–21.
Chen M, Bao W, Aizman R, Huang P, Aspevall O, Gustafsson LE, et al. Activation of extracellular signal-regulated kinase mediates apoptosis induced by uropathogenic Escherichia coli toxins via nitric oxide synthase: protective role of heme oxygenase-1. J Infect Dis 2004; 190: 127–35.
Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998; 91: 3527–61.
Frey EA, Finlay BB . Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb Pathog 1998; 24: 101–9.
McCuskey RS, Urbaschek R, Urbaschek B . The microcirculation during endotoxemia. Cardiovasc Res 1996; 32: 752–63.
Sorimachi H, Ishiura S, Suzuki K . Structure and physiological function of calpains. Biochem J 1997; 328: 721–32.
Sardet C, Counillon L, Franchi A, Pouyssegur J . Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science 1990; 247: 723–6.
Green DR, Reed JC . Mitochondria and apoptosis. Science 1998; 281: 1309–12.
Acknowledgements
We thank Dr Humphrey (Department of Medicine, University of Oklahoma Health Sciences Center) for critically reading this manuscript. This work was funded by Jilin University grant (450060445662, 430504001043, and 430505010272 to Qi-sheng PENG; 4305050102Q1 to Wan-chun SUN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Gm., Zhao, Yx., Zhang, Nn. et al. Amiloride attenuates lipopolysaccharide-accelerated atherosclerosis via inhibition of NHE1-dependent endothelial cell apoptosis. Acta Pharmacol Sin 34, 231–238 (2013). https://doi.org/10.1038/aps.2012.155
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.155
Keywords
This article is cited by
-
Na+-H+ exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis
Nature Communications (2019)