Abstract
Aim:
To investigate the effects of safflor yellow A (SYA), a flavonoid extracted from Carthamus tinctorius L, on cultured rat cardiomyocytes exposed to anoxia/reoxygenation (A/R).
Methods:
Primary cultured neonatal rat cardiomyocytes were exposed to anoxia for 3 h followed by reoxygenation for 6 h. The cell viability was measured using MTT assay. The releases of lactate dehydrogenase (LDH) and creatine kinase (CK), level of malondialdehyde (MDA), and activities of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were analyzed. Hoechst 33258 staining and changes in Bcl-2/Bax ratio and caspase 3 activity were used to examine A/R-induced apoptosis.
Results:
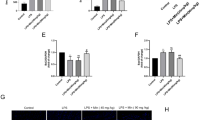
The A/R exposure markedly decreased the viability of cardiomyocytes, suppressed the activities of SOD, GSH, CAT and GSH-Px, and Bcl-2 protein expression. Meanwhile, the A/R exposure markedly increased the release of LDH and CK, and MDA production in the cardiomyocytes, and increased the rate of apoptosis, caspase 3 activity, Bax protein expression. Pretreatment with SYA (40, 60 and 80 nmol/L) concentration-dependently blocked the A/R-induced changes in the cardiomyocytes. Pretreatment of the cardiomyocytes with the antioxidant N-acetylcysteine (NAC, 200 μmol/L) produced protective effects that were comparable to those caused by SYA (80 nmol/L).
Conclusion:
SYA protects cultured rat cardiomyocytes against A/R injury, maybe via inhibiting cellular oxidative stress and apoptosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Reiter RJ, Tan DX . Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res 2003; 58: 10–9.
Goldhaber JI, Weiss JN . Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension 1992; 20: 118–27.
Bolli R, Marban E . Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 1999; 79: 609–34.
Hess ML, Barnhart GR, Crute S, Komwatana P, Krause S, Greenfield LJ . Mechanical and biochemical effects of transient myocardial ischemia. J Surg Res 1979; 26: 175–84.
Limbruno U, Zucchi R, Ronca-Testoni S, Galbani P, Ronca G, Mariani M . Sarcoplasmic reticulum function in the 'stunned' myocardium. J Mol Cell Cardiol 1989; 21: 1063–72.
Jeroudi MO, Hartley CJ, Bolli R . Myocardial reperfusion injury: role of oxygen radicals and potential therapy with antioxidants. Am J Cardiol 1994; 73: 2B–7B.
Braunwald E, Kloner RA . The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 1982; 66: 1146–9.
Vatner DE, Kiuchi K, Manders WT, Vatner SF . Effects of coronary arterial reperfusion on beta-adrenergic receptor-adenylyl cyclase coupling. Am J Physiol 1993; 264: H196–H204.
MacLellan WR, Schneider MD . Death by design. Circ Res 1997; 81: 137–44.
Haunstetter A, Izumo S . Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 1998; 82: 1111–29.
Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK . Free radicals and the heart. J Pharmacol Toxicol Methods 1993; 30: 55–67.
Gustafsson AB, Gottlieb RA . Mechanisms of apoptosis in the heart. J Clin Immunol 2003; 23: 447–59.
Wua L, Qiao H . Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 2007; 14: 652–8.
Li XZ, Liu JX, Shang XH, Fu JH . Protective effects of hydroxysafflor yellow A on acute myocardial ischemia in dogs. Chin Pharmacol Bull 2006; 6: 533–7.
Zheng WC, Chen DB, Li B, Zhang L . The preventive effect safflor yellow on myocardium injury of myocardium ischemic reperfusion in rats. Chin Pharmacol Bull 2003; 19: 1032–4.
Palter R, Lundin RE, Haddon WF . A cathartic lignan glycoside isolated from carthamus tinctorus. Phytochemistry 1972; 11: 2871–4.
Kazuma K, Takahashi T, Sato K, Takeuchi H, Matsumoto T, Okuno T . Quinochalcones and flavonoids from fresh floretsin different cultivars of Carthamus tinctorius L. Biosci Biotechnol Biochem 2000; 64: 1588–99.
Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, et al. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996; 43: 1255–60.
Hirokawa T, Hirokawa S, Yamauchi N, Kataoka T, Woo JT, Nagai K . Immunomodulating activities of polysaccharide fractions from dried safflower petals. Cytotechnology 1997; 25: 205–11.
An XQ, Li YH, Chen J . Separation and identification of safflower yellow A and carthamin from Carthamus tinctorius L. Chin Tradit Herb Drugs 1990; 21: 188–9.
Meselhy MR, Kadota S, Momose Y, Hatakeyama N, Kusai A, Hattori M, et al. Two new quinochalcone yellow pigments from Carthamus tinctorius and Ca2+ antagonistic activity of tinctormine. Chem Pharm Bull 1993; 41: 1796–802.
Yin HB, He ZS . A novel semi-quinone chalcone sharing a pyrrole ring C-glycoside from carthamus tinctorius. Tetrahedrom Lett 2000; 41: 1955–8.
Meselhy MR, Kadota S, Momose Y, Hattori M, Namba T . Tinctormine, a novel Ca2+ antagonist N-containing quinochalcone C-glycoside from Carthamus tinctorius L. Chem Pharm Bull 1992; 40: 3355–7.
Onodera J, Obara H, Hirose R, Matsuba S, Sato N, Suzuki M . The structure of safflomin C, a constituent of safflower. Chem Lett 1989; 9: 1571–4.
Wang CY, Ma HM, Zhang SP . Safflor yellow B suppresses pheochromocytoma cell (PC 12) injury induced by oxidative stress via antioxidant system and Bcl-2 /Bax pathway. Naunyn Schmiedebergs Arch Pharmacol 2009; 380: 135–42.
Xue HY, Wei XB, Ding H . The protective effects of hydroxysafflower yellow A on hypoxia cardiomyocyte. Zhongguo Lao Nian Xue Za Zhi 2007; 7: 140–2.
Wei XB, Liu XQ . Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett 2005; 386: 58–62.
Liu SX, Zhang X, Wang YF, Li XC, Xiang MX, Bian C, et al. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int J Cardiol 2012; 160: 95–101.
Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, et al. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem 1997; 272: 19115–24.
Koyama T, Temma K, Akera T . Reperfusion-induced contracture develops with a decreasing [Ca2+] in single heart cells. Am J Physiol 1991; 261: H1115–22.
Nakano M, Knowlton AA, Dibbs Z, Mann DL . Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation 1998; 97: 1392–400.
Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, et al. Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis 2006; 11: 1453–60.
Turguta G, Enlib Y . Changes in the levels of MDA and GSH in mice serum, liver and spleen after aluminum administration. East J Med 2006; 11: 7–12.
Botsoglou NA, Fletouris DJ . Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem 1994; 42: 1931–7.
Rice-Evans C . Flavonoid antioxidants. Curr Med Chem 2001; 8: 797–807
Amorini AM, Lazzarino G, Galvano F, Fazzina G, Tavazzi B, Galvano G . Cyanidin-3-O-beta-glucopyranoside protects myocardium and erythrocytes from oxygen radical-mediated damages. Free Radic Res 2003; 37: 453–60.
Fantinelli JC, Schinella G, Cingolani HE, Mosca SM . Effects of different fractions of a red wine non-alcoholic extract on ischemia-reperfusion injury. Life Sci 2005; 76: 2721–33.
Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, et al. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci 2007; 80: 1020–32.
Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, et al. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol 2006; 552: 123–30.
Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B . Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 2004; 10: 55–62.
Ji X, Xu Z, Criswell HE, Boysen PG . Propyl paraben inhibits voltage-dependent sodium channels and protects cardiomyocytes from ischemia-reperfusion injury. Life Sci 2004; 74: 3043–52.
Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N-acetylcysteine prevents reactive oxygenspecies-mediated myocardial stress in patients undergoing cardiac surgery: Results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg 2003; 126: 1513–20.
Orhan G, Yapici N, Yuksel M, Sargin M, Senay S, Yalçin AS, et al. Effects of N-acetylcysteine on myocardial ischemia-reperfusion injury in bypass surgery. Heart Vessels 2006; 21: 42–7.
Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K . Comparison of the protective effect of N-Acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci 2008; 106: 571–7.
Hyunsoo K, Sung CY, Tae YL, Daewon J . Discriminative cytotoxicity assessment based on various cellular damages. Toxicol Lett 2009; 184: 13–7.
Corey MJ, Kinders RJ, Brown LG, Vessella RL . A very sensitive coupled luminescent assay for cytotoxicity and complement-mediated lysis. J Immunol Methods 1997; 207: 43–51.
Fu JJ, Huang HQ, Liu JJ, Pi RB, Chen JW, Liu PQ . Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol 2007; 568: 213–21.
Wu Y, Yuan BX . Effects of Qiangxin capsules on myocardial reperfusion arrhythmias in rats. North Phar J 2001; 16: 23–4.
Kehrer JP, Smith CV . In: Natural antioxidants in human health and disease. Free radicals in biology: sources, reactivates, and roles in the etiology of human diseases. San Diego: CA, USA; 1994. p 32.
Das DK, Maulik N . Antioxidant effectiveness in ischemia/reperfusion tissue injury. Methods Enzymol 1994; 233: 601–10.
Lefer DJ, Granger DN . Oxidative stress and cardiac disease. Am J Med 2000; 109: 315–23.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J . Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006; 160: 1–40.
Tengattini S, Russel J . Cardiovascular diseases: protective effects of melatonin. J Pineal Res 2008; 44: 16–25.
Gottlib RA, Engler RL . Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci 1999; 30: 412–26.
Majno G, Joris I . Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146: 3–15.
Kumar D, Lou HQ, Singal PK . Oxidative stress and apoptosis in heart dysfunction. Herz 2002; 27: 662–8.
Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H . Expression of Bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation 1996; 94: 1506–12.
Hanada M, Aime-Sempe C, Sato T, Reed JC . Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem 1995; 270: 11962–9.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD . The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997; 275: 1132–6.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275: 1129–32.
Tamm I, Schriever F, Dörken B . Apoptosis: implications of basic research for clinical oncology. Lancet Oncol 2001; 2: 33–42.
Oubrahim H, Stadtman ER, Chock PB . Mitochondria play no roles in Mn(II) induced apoptosis in HeLa cells. Proc Natl Acad Sci U S A 2001; 98: 9505–10.
Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 2006; 311: 847–51.
Nicholson DW, Ali A, Thornberry NA, Vai llancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995; 376: 37–43.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No 81173514 and 81001673), the “13115” Technology Innovation Project of Shaanxi Province (No 2010ZDKG-62), Xijing Research Boosting Program (No XJZT10D02) and Excellent Civil Service Training Fund of the Fourth Military Medical University (No 4138C4IDK6). The authors thank colleagues in the New Drug Research and Development Center of Xijing Hospital for their technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Duan, Jl., Wang, Jw., Guan, Y. et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin 34, 487–495 (2013). https://doi.org/10.1038/aps.2012.185
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.185
Keywords
This article is cited by
-
Safflor Yellow A Protects Beas-2B Cells Against LPS-Induced Injury via Activating Nrf2
Revista Brasileira de Farmacognosia (2023)
-
Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress
Acta Pharmacologica Sinica (2016)
-
Pretreatment with low-dose gadolinium chloride attenuates myocardial ischemia/reperfusion injury in rats
Acta Pharmacologica Sinica (2016)
-
Hydroxysafflor yellow A alleviates myocardial ischemia/reperfusion in hyperlipidemic animals through the suppression of TLR4 signaling
Scientific Reports (2016)