Abstract
Aim:
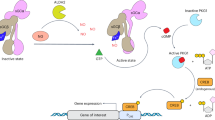
To investigate whether NO over-production in rat mesangial cells cultured in high glucose (HG) is related to activation of the TGF-β1/PI3K/Akt pathway.
Methods:
Rat mesangial cells line (HBZY-1) was exposed to HG (24.44 mmol/L) or H2O2 (10 μmol/L) for 16 h. NO release was quantified using the Griess assay. The TGF-β1 level was measured using ELISA. The protein expression of p-Akt, t-Akt, Bim, and iNOS was examined by Western blotting. The mRNA levels of TGF-β1 and Bim were measured using RT-PCR. The cell proliferation rate was estimated using a BrdU incorporation assay.
Results:
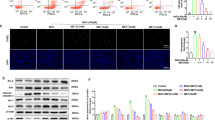
Treatment of the cells with HG, H2O2, or TGF-β1 (5 ng/mL) significantly increased the NO level that was substantially inhibited by co-treatment with the NADPH oxidase inhibitor diphenylene iodonium (DPI), TGF-β1 inhibitor SB431542, or PI3K inhibitor LY294002. Both HG and H2O2 significantly increased the protein and mRNA levels of TGF-β1 in the cells, and HG-induced increases of TGF-β1 protein and mRNA were blocked by co-treatment with DPI. Furthermore, the treatment with HG or H2O2 significantly increased the expression of phosphorylated Akt and iNOS and cell proliferation rate, which was blocked by co-treatment with DPI, SB431542, or LY294002. Moreover, the treatment with HG or H2O2 significantly inhibited Bim protein and mRNA expression, which was reversed by co-treatment with DPI, SB431542, or LY294002.
Conclusion:
The results demonstrate that high glucose causes oxidative stress and NO over-production in rat mesangial cells in vitro via decreasing Bim and increasing iNOS, which are at least partially mediated by the TGF-β1/PI3K/Akt pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008; 233: 4–11.
Ha H, Lee HB . Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology (Carlton) 2005; Suppl: S7–10.
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 2010; 299: F1348–58.
Jaimes EA, Hua P, Tian RX, Raij L . Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol 2009; 298: F125–32.
Brownlee M . The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–25.
Yin X, Zhang Y, Yu J, Zhang B, Shen J, Qiu J, et al. The antioxidative effects of astragalus saponin I protect against development of early diabetic nephropathy. J Pharmacol Sci 2006; 101: 166–73.
Yin X, Zhang Y, Yu J, Zhang B, Shen J, Qiu J . Protective effects of bendazac lysine against development of diabetic nephropathy. Chin J Clin Pharmacol Ther 2007; 12: 552–7.
Lu Q, Yin XX, Wang JY, Gao YY, Pan YM . Effects of Ginkgo biloba on prevention of development of experimental diabetic nephropathy in rats. Acta Pharmacol Sin 2007; 28: 818–28.
Levin-Iaina N, Iaina A, Raz I . The emerging role of NO and IGF-1 in early renal hypertrophy in STZ-induced diabetic rats. Diabetes Metab Res Rev 2011; 27: 235–43.
Noh H, Ha H, Yu MR, Kang SW, Choi KH, Han DS, et al. High glucose increases inducible NO production in cultured rat mesangial cells. Possible role in fibronectin production. Nephron 2002; 90: 78–85.
Erdely A, Freshour G, Maddox DA, Olson JL, Samsell L, Baylis C . Renal disease in rats with type 2 diabetes is associated with decreased renal nitric oxide production. Diabetologia 2004; 47: 1672–6.
Keynan S, Hirshberg B, Levin-Iaina N, Wexler ID, Dahan R, Reinhartz E, et al. Renal nitric oxide production during the early phase of experimental diabetes mellitus. Kidney Int 2000; 58: 740–7.
Komers R, Anderson S . Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol 2003; 284: F1121–37.
Prabhakar SS . Role of nitric oxide in diabetic nephropathy. Semin Nephrol 2004; 24: 333–44.
Lee HY, Noh HJ, Gang JG, Xu ZG, Jeong HJ, Kang SW, et al. Inducible nitric oxide synthase (iNOS) expression is increased in lipopolysaccharide (LPS)-stimulated diabetic rat glomeruli: effect of ACE inhibitor and angiotensin II receptor blocker. Yonsei Med J 2002; 43: 183–92.
Shultz PJ, Archer SL, Rosenberg ME . Inducible nitric oxide synthase mRNA and activity in glomerular mesangial cells. Kidney Int 1994; 46: 683–9.
Sefi M, Fetoui H, Soudani N, Chtourou Y, Makni M, Zeghal N . Artemisia campestris leaf extract alleviates early diabetic nephropathy in rats by inhibiting protein oxidation and nitric oxide end products. Pathol Res Pract 2012; 208: 157–62.
Anjaneyulu M, Chopra K . Effect of irbesartan on the antioxidant defence system and nitric oxide release in diabetic rat kidney. Am J Nephrol 2004; 24: 488–96.
Levine DZ . Hyperfiltration, nitric oxide, and diabetic nephropathy. Curr Hypertens Rep 2006; 8: 153–7.
Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL . Sustained Wnt/β-Catenin signaling rescues high glucose induction of transforming growth factor-β1-mediated renal fibrosis. Am J Med Sci 2012; 344: 374–82.
Du J, Wang L, Liu L, Fan Q, Yao L, Cui Y, et al. IFN-γ suppresses the high glucose-induced increase in TGF-β1 and CTGF synthesis in mesangial cells. Pharmacol Rep 2011; 63: 1137–44.
Uttarwar L, Gao B, Ingram AJ, Krepinsky JC . SREBP-1 activation by glucose mediates TGF-β upregulation in mesangial cells. Am J Physiol Renal Physiol 2012; 302: F329–41.
Yin X, Zhang Y, Wu H, Zhu X, Zheng X, Jiang S, et al. Protective effects of Astragalus saponin I on early stage of diabetic nephropathy in rats. J Pharmacol Sci 2004; 95: 256–66.
Wang JY, Yin XX, Wu YM, Tang DQ, Gao YY, Wan MR, et al. Ginkgo biloba extract suppresses hypertrophy and extracellular matrix accumulation in rat mesangial cells. Acta Pharmacol Sin 2006; 27: 1222–30.
Ji L, Yin XX, Wu ZM, Wang JY, Lu Q, Gao YY . Ginkgo biloba extract prevents glucose-induced accumulation of ECM in rat mesangial cells. Phytother Res 2009; 23: 477–85.
Runyan CE, Schnaper HW, Poncelet AC . The phosphatidylinositol 3-kinase/Akt pathway enhances smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 2004; 279: 2632–9.
Qureshi HY, Ahmad R, Sylvester J, Zafarullah M . Requirement of phosphatidylinositol 3-kinase/Akt signaling pathway for regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-beta in human chondrocytes. Cell Signal 2007; 19: 1643–51.
Yi JY, Shin I, Arteaga CL . Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem 2005; 280: 10870–6.
Ghosh Choudhury G, Abboud HE . Tyrosine phosphorylation-dependent PI3 kinase/Akt signal transduction regulates TGF beta-induced fibronectin expression in mesangial cells. Cell Signal 2004; 16: 31–41.
Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 2003; 278: 49795–805.
Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 2005; 24: 2317–29.
Urbich C, Knau A, Fichtlscherer S, Walter DH, Brühl T, Potente M, et al. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J 2005; 19: 974–6.
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002; 419: 316–21.
Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, et al. Role of the Akt/FoxO3a pathway in TGF-β1-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 2006; 17: 3325–35.
Li M, Chiu JF, Mossman BT, Fukagawa NK . Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem 2006; 281: 40429–39.
Liu H, Luo LL, Qian YS, Fu YC, Sui XX, Geng YJ, et al. FOXO3a is involved in the apoptosis of naked oocytes and oocytes of primordial follicles from neonatal rat ovaries. Biochem Biophys Res Commun 2009; 381: 722–7.
Kozuma Y, Ninomiya H, Murata S, Kono T, Mukai HY, Kojima H . The pro-apoptotic BH3-only protein Bim regulates cell cycle progression of hematopoietic progenitors during megakaryopoiesis. J Thromb Haemost 2010; 8: 1088–97.
Cash TP, Gruber JJ, Hartman TR, Henske EP, Simon MC . Loss of the Birt-Hogg-Dubé tumor suppressor results in apoptotic resistance due to aberrant TGFβ-mediated transcription. Oncogene 2011; 30: 2534–46.
Hoshino Y, Katsuno Y, Ehata S, Miyazono K . Autocrine TGF-β protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem 2011; 149: 55–65.
Oussaief L, Ramírez V, Hippocrate A, Arbach H, Cochet C, Proust A, et al. NF-kappaB-mediated modulation of inducible nitric oxide synthase activity controls induction of the Epstein-Barr virus productive cycle by transforming growth factor beta 1. J Virol 2011; 85: 6502–12.
Hamby ME, Hewett JA, Hewett SJ . TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia 2006; 54: 566–77.
Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant 2011; 26: 2119–26.
Sheibani N, Morrison ME, Gurel Z, Park S, Sorenson CM . BIM deficiency differentially impacts the function of kidney endothelial and epithelial cells through modulation of their local microenvironment. Am J Physiol Renal Physiol 2012; 302: F809–19.
Lu Q, Zhai Y, Cheng Q, Liu Y, Gao X, Zhang T, et al. The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp Physiol 2012. DOI: 10.1113/expphysiol.2012.068361.
Wolf G . Molecular mechanisms of diabetic mesangial cell hypertrophy: a proliferation of novel factors. J Am Soc Nephrol 2002; 13: 2611–3.
Acknowledgements
We thank Miss Hao GUO for help with the manuscript. The work was supported by the National Natural Science Foundation of China (No 81173104), the Jiangsu University Natural Science Foundation of China (No 11KJD310004), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, Yp., Lu, Q., Liu, Yw. et al. Over-production of nitric oxide by oxidative stress-induced activation of the TGF-β1/PI3K/Akt pathway in mesangial cells cultured in high glucose. Acta Pharmacol Sin 34, 507–514 (2013). https://doi.org/10.1038/aps.2012.207
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.207
Keywords
This article is cited by
-
MiR-29b expression is altered in crescent formation of HSPN and accelerates Ang II-induced mesangial cell activation
World Journal of Pediatrics (2020)
-
Ameliorative Effect of Selenomethionine on Cadmium-Induced Hepatocyte Apoptosis via Regulating PI3K/AKT Pathway in Chickens
Biological Trace Element Research (2020)
-
Seismic rock physical modelling for gas hydrate-bearing sediments
Science China Earth Sciences (2018)
-
Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZB×NZW F1 mice via interference with TLR-mediated APC response
Acta Pharmacologica Sinica (2014)
-
Selective α1B- and α1D-adrenoceptor antagonists suppress noradrenaline-induced activation, proliferation and ECM secretion of rat hepatic stellate cells in vitro
Acta Pharmacologica Sinica (2014)