Abstract
Aim:
To determine whether electrical stimulation of caudal medial prefrontal cortex (mPFC) as conditioned stimulus (CS) paired with airpuff unconditioned stimulus (US) was sufficient for establishing eyeblink conditioning in guinea pigs, and whether it was dependent on cerebellar interpositus nucleus.
Methods:
Thirty adult guinea pigs were divided into 3 conditioned groups, and trained on the delay eyeblink conditioning, short-trace eyeblink conditioning, and long-trace eyeblink conditioning paradigms, respectively, in which electrical stimulation of the right caudal mPFC was used as CS and paired with corneal airpuff US. A pseudo conditioned group of another 10 adult guinea pigs was given unpaired caudal mPFC electrical stimulation and the US. Muscimol (1 μg in 1 μL saline) and saline (1 μL) were infused into the cerebellar interpositus nucleus of the animals through the infusion cannula on d 11 and 12, respectively.
Results:
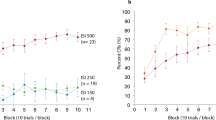
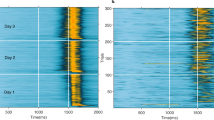
The 3 eyeblink conditioning paradigms have been successfully established in guinea pigs. The animals acquired the delay and short-trace conditioned responses more rapidly than long-trace conditioned responses. Muscimol infusion into the cerebellar interpositus nucleus markedly impaired the expression of the 3 eyeblink conditioned responses.
Conclusion:
Electrical stimulation of caudal mPFC is effective CS for establishing eyeblink conditioning in guinea pigs, and it is dependent on the cerebellar interpositus nucleus.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Christian KM, Thompson RF . Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 2003; 10: 427–55.
Christian KM, Thompson RF . Long-term storage of an associative memory trace in the cerebellum. Behav Neurosci 2005; 119: 526–37.
Thompson RF . In search of memory traces. Annu Rev Psychol 2005; 56: 1–23.
Weeks AC, Connor S, Hinchcliff R, LeBoutillier JC, Thompson RF, Petit TL . Eye-blink conditioning is associated with changes in synaptic ultrastructure in the rabbit interpositus nuclei. Learn Mem 2007; 14: 385–9.
Green JT, Arenos JD . Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol Learn Mem 2007; 87: 269–84.
Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD . Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem 2009; 16: 86–95.
Halverson HE, Lee I, Freeman JH . Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. J Neurosci 2010; 30: 8787–96.
Halverson HE, Freeman JH . Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiol Learn Mem 2010; 93: 92–8.
Halverson HE, Poremba A, Freeman JH . Medial auditory thalamus inactivation prevents acquisition and retention of eyeblink conditioning. Learn Mem 2008; 15: 532–8.
Freeman JH, Halverson HE, Hubbard EM . Inferior colliculus lesions impair eyeblink conditioning in rats. Learn Mem 2007; 14: 842–6.
Campolattaro MM, Halverson HE, Freeman JH . Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learn Mem 2007; 14: 152–9.
Halverson HE, Freeman JH . Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behav Neurosci 2006; 120: 880–7.
Oswald BB, Knuckley B, Maddox SA, Powell DA . Ibotenic acid lesions to ventrolateral thalamic nuclei disrupts trace and delay eyeblink conditioning in rabbits. Behav Brain Res 2007; 179: 111–7.
Halverson HE, Freeman JH . Ventral lateral geniculate input to the medial pons is necessary for visual eyeblink conditioning in rats. Learn Mem 2010; 17: 80–5.
Simon B, Knuckley B, Churchwell J, Powell DA . Post–training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci 2005; 25: 10740–6.
Oswald BB, Maddox SA, Powell DA . Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR? Behav Neurosci 2008; 122: 841–8.
Oswald BB, Maddox SA, Tisdale N, Powell DA . Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: a study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem 2010; 93: 37–45.
Powell DA, Churchwell J, Burriss L . Medial prefrontal lesions and Pavlovian eyeblink and heart rate conditioning: effects of partial reinforcement on delay and trace conditioning in rabbits (Oryctolagus cuniculus). Behav Neurosci 2005; 119: 180–9.
Weible AP, McEchron MD, Disterhoft JF . Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci 2000; 114: 1058–67.
Weible AP, Weiss C, Disterhoft JF . Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. J Neurophysiol 2003; 90: 599–612.
Takehara-Nishiuchi K, Kawahara S, Kirino Y . NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn Mem 2005; 12: 606–14.
Weiss C, Bouwmeester H, Power JM, Disterhoft JF . Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res 1999; 99: 123–32.
Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ . The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem 2001; 76: 447–61.
Tseng W, Guan R, Disterhoft JF, Weiss C . Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus 2004; 14: 58–65.
Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ . Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 1986; 100: 729–44.
Moyer JR Jr, Deyo RA, Disterhoft JF . Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 1990; 104: 243–52.
Hu B, Yang L, Huang LS, Chen H, Zeng Y, Feng H, et al. Effect of cerebellar reversible inactivations on the acquisition of trace conditioned eyeblink responses in guinea pigs: comparison of short and long trace intervals. Neurosci Lett 2009; 459: 41–5.
Hu B, Lin X, Huang LS, Yang L, Feng H, Sui JF . Involvement of the ipsilateral and contralateral cerebellum in the acquisition of unilateral classical eyeblink conditioning in guinea pigs. Acta Pharmacol Sin 2009; 30: 141–52.
Pakaprot N, Kim S, Thompson RF . The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci 2009; 123: 54–61.
Takehara K, Kawahara S, Kirino Y . Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 2003; 23: 9897–905.
Woodruff-Pak DS, Lavond DG, Thompson RF . Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res 1985; 348: 249–60.
Freeman JH, Duffel JW . Eyeblink conditioning using cochlear nucleus stimulation as a conditioned stimulus in developing rats. Dev Psychobiol 2008; 50: 640–6.
Knowlton BJ, Thompson RF . Conditioning using a cerebral cortical conditioned stimulus is dependent on the cerebellum and brain stem circuitry. Behav Neurosci 1992; 106: 509–17.
Halverson HE, Hubbard EM, Freeman JH . Stimulation of the lateral geniculate, superior colliculus, or visual cortex is sufficient for eyeblink conditioning in rats. Learn Mem 2009; 16: 300–7.
Freeman JH Jr, Rabinak CA . Eyeblink conditioning in rats using pontine stimulation as a conditioned stimulus. Integr Physiol Behav Sci 2004; 39: 180–91.
Rosen DJ, Steinmetz JE, Thompson RF . Classical discrimination conditioning of the rabbit's eyelid response using pontine stimulation as a conditioned stimulus. Behav Neural Biol 1989; 52: 51–62.
Steinmetz JE, Lavond DG, Thompson RF . Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse 1989; 3: 225–33.
Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF . Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav Neurosci 1986; 100: 878–87.
Steinmetz JE, Rosen DJ, Woodruff-Pak DS, Lavond DG, Thompson RF . Rapid transfer of training occurs when direct mossy fiber stimulation is used as a conditioned stimulus for classical eyelid conditioning. Neurosci Res 1986; 3: 606–16.
Shinkman PG, Swain RA, Thompson RF . Classical conditioning with electrical stimulation of cerebellum as both conditioned and unconditioned stimulus. Behav Neurosci 1996; 110: 914–21.
Jirenhed DA, Hesslow G . Time course of classically conditioned Purkinje cell response is determined by initial part of conditioned stimulus. J Neurosci 2011; 31: 9070–4.
Poulos AM, Thompson RF . Timing of conditioned responses utilizing electrical stimulation in the region of the interpositus nucleus as a CS. Integr Physiol Behav Sci 2004; 39: 83–94.
Jimenez-Diaz L, Navarro-Lopez Jde D, Gruart A, Delgado-Garcia JM . Role of cerebellar interpositus nucleus in the genesis and control of reflex and conditioned eyelid responses. J Neurosci 2004; 24: 9138–45.
Leal-Campanario R, Delgado-Garcia JM, Gruart A . Microstimulation of the somatosensory cortex can substitute for vibrissa stimulation during Pavlovian conditioning. Proc Natl Acad Sci U S A 2006; 103: 10052–7.
Woody CD, Yarowsky PJ . Conditioned eye blink using electrical stimulation of coronal-precruciate cortex as conditional stimulus. J Neurophysiol 1972; 35: 242–52.
Troncoso J, Munera A, Delgado-Garcia JM . Classical conditioning of eyelid and mystacial vibrissae responses in conscious mice. Learn Mem 2004; 11: 724–6.
Goldman-Rakic PS . Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res 1990; 85: 325–35; discussion 35–6.
Goldman-Rakic PS . Cellular basis of working memory. Neuron 1995; 14: 477–85.
Fuster JM . The prefrontal cortex — an update: time is of the essence. Neuron 2001; 30: 319–33.
Corbit LH, Balleine BW . The role of prelimbic cortex in instrumental conditioning. Behav Brain Res 2003; 146: 145–57.
Powell DA, Skaggs H, Churchwell J, McLaughlin J . Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 2001; 115: 1029–38.
Doty RW, Larsen RM, Ruthledge LT Jr . Conditioned reflexes established to electrical stimulation of cat cerebral cortex. J Neurophysiol 1956; 19: 401–15.
Simon B, Knuckley B, Churchwell J, Powell DA . Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J Neurosci 2005; 25: 10740–6.
Oswald BB, Maddox SA, Powell DA . Prefrontal control of trace eyeblink conditioning in rabbits: role in retrieval of the CR? Behav Neurosci 2008; 122: 841–8.
Kronforst-Collins MA, Disterhoft JF . Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem 1998; 69: 147–62.
Chachich M, Powell DA . Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett 1998; 257: 151–4.
Powell DA, Skaggs H, Churchwell J, McLaughlin J . Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 2001; 115: 1029–38.
Leal–Campanario R, Fairen A, Delgado-Garcia JM, Gruart A . Electrical stimulation of the rostral medial prefrontal cortex in rabbits inhibits the expression of conditioned eyelid responses but not their acquisition. Proc Natl Acad Sci U S A 2007; 104: 11459–64.
McLaughlin J, Skaggs H, Churchwell J, Powell DA . Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behav Neurosci 2002; 116: 37–47.
Rapisarda C, Bacchelli B . The brain of the guinea pig in stereotaxic coordinates. Arch Sci Biol (Bologna) 1977; 61: 1–37.
Martin JH . Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 1991; 127: 160–4.
Martin JH, Ghez C . Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 1999; 86: 145–59.
Sarihi A, Motamedi F, Naghdi N, Rashidy-Pour A . Lidocaine reversible inactivation of the median raphe nucleus has no effect on reference memory but enhances working memory versions of the Morris water maze task. Behav Brain Res 2000; 114: 1–9.
Hu B, Chen H, Yang L, Tao ZF, Yan J, Zhang YH, et al. Changes of synaptic ultrastructure in the guinea pig interpositus nuclei associate with response magnitude and timing after trace eyeblink conditioning. Behav Brain Res 2012; 226: 529–37.
Kotani S, Kawahara S, Kirino Y . Trace eyeblink conditioning in decerebrate guinea pigs. Eur J Neurosci 2003; 17: 1445–54.
Green JT, Arenos JD . Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol Learn Mem 2007; 87: 269–84.
Vogt LJ, Vogt BA, Sikes RW . Limbic thalamus in rabbit: architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA, and opioid receptors. J Comp Neurol 1992; 319: 205–17.
Debiec J, LeDoux JE, Nader K . Cellular and systems reconsolidation in the hippocampus. Neuron 2002; 36: 527–38.
Powell DA, Churchwell J . Mediodorsal thalamic lesions impair trace eyeblink conditioning in the rabbit. Learn Mem 2002; 9: 10–7.
Hu B, Chen H, Feng H, Zeng Y, Yang L, Fan ZL, et al. Disrupted topography of the acquired trace-conditioned eyeblink responses in guinea pigs after suppression of cerebellar cortical inhibition to the interpositus nucleus. Brain Res 2010; 1337: 41–55.
Garcia KS, Mauk MD . Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 1998; 37: 471–80.
Steinmetz JE . Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res 2000; 110: 13–24.
Koutalidis O, Foster A, Weisz DJ . Parallel pathways can conduct visual CS information during classical conditioning of the NM response. J Neurosci 1988; 8: 417–27.
Prokasy WF, Kesner RP, Calder LD . Posttrial electrical stimulation of the dorsal hippocampus facilitates acquisition of the nictitating membrane response. Behav Neurosci 1983; 97: 890–6.
Kalmbach BE, Ohyama T, Mauk MD . Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. J Neurophysiol 2010; 104: 627–40.
Wada N, Kishimoto Y, Watanabe D, Kano M, Hirano T, Funabiki K, et al. Conditioned eyeblink learning is formed and stored without cerebellar granule cell transmission. Proc Natl Acad Sci U S A 2007; 104: 16690–5.
Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD . Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol 2012; 107: 50–64.
Okuda Y, Shikata H, Song WJ . A train of electrical pulses applied to the primary auditory cortex evokes a conditioned response in guinea pigs. Neurosci Res 2011; 71: 103–6.
Bao S, Chen L, Thompson RF . Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behav Neurosci 2000; 114: 254–61.
Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF . Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc Natl Acad Sci U S A 1987; 84: 3531–5.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (No 30771769). We thank the assistance of De-ying CHEN in preparing the photomicrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Gy., Yao, J., Fan, Zl. et al. Classical eyeblink conditioning using electrical stimulation of caudal mPFC as conditioned stimulus is dependent on cerebellar interpositus nucleus in guinea pigs. Acta Pharmacol Sin 33, 717–727 (2012). https://doi.org/10.1038/aps.2012.32
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.32
Keywords
This article is cited by
-
Reevaluating the ability of cerebellum in associative motor learning
Scientific Reports (2019)
-
Electrical Stimulation Normalizes c-Fos Expression in the Deep Cerebellar Nuclei of Depressive-like Rats: Implication of Antidepressant Activity
The Cerebellum (2017)
-
Optogenetic stimulation of mPFC pyramidal neurons as a conditioned stimulus supports associative learning in rats
Scientific Reports (2015)
-
Prefrontal Control of Cerebellum-Dependent Associative Motor Learning
The Cerebellum (2014)