Abstract
Aim:
Plasminogen activator inhibitor-1 (PAI-1) is involved in the progression of pulmonary fibrosis. The present study was undertaken to examine the effects on pulmonary fibrosis of silencing PAI-1 expression with small interfering RNA (siRNA) and to assess the possible underlying mechanisms.
Methods:
Male Wistar rats were subjected to intratracheal injection of bleomycin (BLM, 5 mg/kg, 0.2 mL) to induce pulmonary fibrosis. Histopathological changes of lung tissue were examined with HE or Masson's trichrome staining. The expression levels of α-smooth muscle actin (α-SMA), collagen type-I and type-III, caspase-3, as well as p-ERK1/2 and PI3K/Akt in the lung tissue were evaluated using imunohistochemistry and Western blot analyses. The fibroblasts isolated from BLM-induced fibrotic lung tissue were cultured and transfected with pcDNA-PAI-1 or PAI-1siRNA. The expression level of PAI-1 in the fibroblasts was measured using real time RT-PCR and Western blot analysis. The fibroblast proliferation was evaluated using MTT assay.
Results:
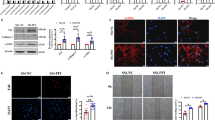
Intratracheal injection of PAI-1-siRNA (7.5 nmoL/0.2 mL) significantly alleviated alveolitis and collagen deposition, reduced the expression of PAI-1, α-SMA, collagen type-I and collagen type-III, and increased the expression of caspase-3 in BLM-induced fibrotic lung tissue. In consistence with the in vivo results, the proliferation of the cultured fibroblasts from BLM-induced fibrotic lung tissue was inhibited by transfection with PAI-1-siRNA, and accelerated by overexpression of PAI-1 by transfection with pcDNA-PAI-1. The expression of caspase-3 was increased as a result of PAI-1 siRNA transfection, and decreased after transfection with pcDNA-PAI-1. In addition, the levels of p-ERK1/2 and PI3K/Akt in the fibrogenic lung tissue were reduced after treatment with PAI-1siRNA.
Conclusion:
The data demonstrate that PAI-1 siRNA inhibits alveolitis and pulmonary fibrosis in BLM-treated rats via inhibiting the proliferation and promoting the apoptosis of fibroblasts. Suppression ERK and AKT signalling pathways might have at least partly contributed to this process. Targeting PAI-1 is a promising therapeutic strategy for pulmonary fibrosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Doubková M, Skhicková J . Idiopathic pulmonary fibrosis. Vnitr Lek 2005; 51: 1375–84.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G . The myofibroblast: one function, multiple origins. Am J Pathol 2007; 170: 1807–16.
Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N . The urokinase-system-role of cell proliferation and apoptosis. Histol Histopathol 2008; 23: 227–36.
Bueno M, Salgado S, Beas-Zárate C, Armendariz-Borunda J . Urokinase-type plasminogen activator gene therapy in liver cirrhosis is mediated by collagens gene expression down-regulation and up-regulation of MMPs, HGF and VEGF. J Gene Med 2006; 8: 1291–9.
Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, et al. The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol 2004; 164: 1091–8.
Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, et al. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med 1990; 322: 890–7.
Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, et al. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 1996; 97: 232–7.
Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, et al. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor-1. Am J Pathol 2003; 163: 445–52.
Hu PF, Zhu YW, Zhong W, Chen YX, Lin Y, Zhang X, et al. Inhibition of plasminogen activator inhibitor-1 expression by siRNA in rat hepatic stellate cells. J Gastroenterol Hepatol 2008; 23: 1917–25.
Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, et al. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax 2010; 65: 334–40.
Durcan N, Murphy C, Cryan SA . Inhalable siRNA: potential as a therapeutic agent in the lungs. Mol Pharm 2008; 5: 559–66.
Castanotto D, Rossi1 JJ . The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009; 457: 426–33.
Thannickal VJ, Horowitz JC . Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006; 3: 350–6.
Hinz B, Gabbiani G . Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost 2003; 90: 993–1002.
Phan SH . The myofibroblast in pulmonary fibrosis. Chest 2002; 122: 286S–9S.
Offersen BV, Nielsen BS, Høyer-Hansen G, Rank F, Hamilton-Dutoit S, Overgaard J, et al. The myofibroblast is the predominant plasminogen activator inhibitor-1-expressing cell type in human breast carcinomas. Am J Pathol 2003; 163: 1887–99.
Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK . Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Bio Cell 2007; 18: 2716–27.
Hu PF, Chen H, Zhong W, Lin Y, Zhang X, Chen YX, et al. Adenovirus–mediated transfer of siRNA against PAI-1 mRNA ameliorates hepatic fibrosis in rats. J Hepatol 2009; 51: 102–13.
Dellas C, Loskutoff DJ . Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost 2005; 93: 631–40.
Binder BR, Mihaly J, Prager GW . UPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost 2007; 97: 336–42.
Balsara RD, Ploplis VA . Plasminogen activator inhibitor-1: the double-edged sword in apoptosis. Thromb Haemost 2008; 100: 1029–36.
Lademann UA, Rømer MU . Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1). Thromb Haemost 2008; 100: 1041–6.
Al-Fakhri N, Chavakis T, Schmidt-Wöll T, Huang B, Cherian SM, Bobryshev YV, et al. Induction of apoptosis in vascular cells by plasminogen activator inhibitor-1 and high molecular weight kininogen correlates with their anti-adhesive properties. Biol Chem 2003; 384: 423–35.
Chen SC, Henry DO, Reczek PR, Wong MK . Plasminogen activator inhibitor-1 inhibits prostate tumor growth through endothelial apoptosis. Mol Cancer Ther 2008; 7: 1227–36.
Rømer MU, Larsen L, Offenberg H, Brünner N, Lademann UA . Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the PI3K/Akt cell survival pathway. Neoplasia 2008; 10: 1083–91.
Chen Y, Budd RC, Kelm RJ Jr, Sobel BE, Schneider DJ . Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol 2006; 26: 1777–83.
Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, et al. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 2010; 120: 1950–60.
Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science 2006; 311: 656–9.
Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ . Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 2008; 38: 78–87.
Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L . ERK (MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38 (SAPK). Cancer Res 2003; 63: 1684–95.
Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, et al. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol 2002; 159: 1061–70.
Kiian I, Tkachuk N, Haller H, Dumler I . Urokinase-induced migration of human vascular smooth muscle cells requires coupling of the small GTPases RhoA and Rac1 to the Tyk2/PI3-K signalling pathway. Thromb Haemost 2003; 89: 904–14.
Balsara RD, Castellino FJ, Ploplis VA . A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. J Biol Chem 2006; 281: 22527–36.
Zhong W, Shen WF, Ning BF, Hu PF, Lin Y, Yue HY, et al. Inhibition of extracellular signal-regulated kinase 1 by adenovirus mediated small interfering RNA attenuates hepatic fibrosis in rats. Hepatology 2009; 50: 1524–36.
Acknowledgements
We gratefully acknowledge Dr Fang WANG for providing the plasmid pcDNA-PAI-1 and the vector pcDNA3.1; Dr Wen-xin WU and Cui-qing MA for their technical assistance; and Madam Li-Mei LIU for her assistance with the writing.
This work was funded by the National Natural Science Foundation of China (No: 30770738; No: 31040036; No: 81000477), the Key Base Research Project of Hebei Province (No: 11966121D), and the Natural Science Foundation of Hebei Province (No: C2009001161).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Yp., Li, Wb., Wang, Wl. et al. siRNA against plasminogen activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis in rats. Acta Pharmacol Sin 33, 897–908 (2012). https://doi.org/10.1038/aps.2012.39
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.39
Keywords
This article is cited by
-
Asarinin attenuates bleomycin-induced pulmonary fibrosis by activating PPARγ
Scientific Reports (2023)
-
TRPM7 restrains plasmin activity and promotes transforming growth factor-β1 signaling in primary human lung fibroblasts
Archives of Toxicology (2022)