Abstract
Aim:
Osteopontin (OPN), a multifunctional protein, has been reported to be protoxicant in acetaminophen hepatotoxicity. In this study, the mechanisms underlying the detrimental role of OPN in acetaminophen toxicity were explored.
Methods:
Male C57BL/6 (wild-type, WT) and OPN−/− mice were administered with acetaminophen (500 mg/kg, ip). After the treatment, serum transaminase (ALT), as well as OPN expression, histology changes, oxidative stress and inflammation response in liver tissue were studied. Freshly isolated hepatocytes of WT and OPN−/− mice were prepared.
Results:
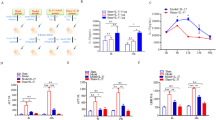
Acetaminophen administration significantly increased OPN protein level in livers of WT mice. OPN expression was mainly localized in hepatic macrophages 6 h after the administration. In OPN−/− mice, acetaminophen-induced serum ALT release was reduced, but the centrilobular hepatic necrosis was increased. In OPN−/− mice, the expression of CYP2E1 and CYP1A2 in livers was significantly increased; GSH depletion and lipid peroxidation in livers were enhanced. On the other hand, OPN−/− mice exhibited less macrophage and neutrophil infiltration and reduced expression of proinflammatory cytokines TNF-α and IL-1α in livers. An anti-OPN neutralizing antibody significantly reduced acetaminophen-induced serum ALT level and inflammatory infiltration in livers of WT mice.
Conclusion:
OPN plays a dual role in acetaminophen toxicity: OPN in hepatocytes inhibits acetaminophen metabolism, while OPN in macrophages enhances acetaminophen toxicity via recruitment of inflammatory cells and production of proinflammatory cytokines.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364–72.
Bessems JG, Vermeulen NP . Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001; 31: 55–138.
Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos 2005; 33: 449–57.
Chen C, Krausz KW, Idle JR, Gonzalez FJ . Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem 2008; 283: 4543–59.
Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ . Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem 1996; 271: 12063–7.
Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, et al. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol 1998; 152: 193–9.
Guo GL, Moffit JS, Nicol CJ, Ward JM, Aleksunes LA, Slitt AL, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci 2004; 82: 374–80.
Rangaswami H, Bulbule A, Kundu GC . Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 2006; 16: 79–87.
Ishijima M, Tsuji K, Rittling SR, Yamashita T, Kurosawa H, Denhardt DT, et al. Osteopontin is required for mechanical stress-dependent signals to bone marrow cells. J Endocrinol 2007; 193: 235–43.
Baliga SS, Merrill GF, Shinohara ML, Denhardt DT . Osteopontin expression during early cerebral ischemia-reperfusion in rats: enhanced expression in the right cortex is suppressed by acetaminophen. PLoS One 2011; 6: e 14568.
Wang KX, Denhardt DT . Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 2008; 19: 333–45.
Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000; 287: 860–4.
Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S . Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leuk Biol 2002; 72: 752–61.
Kawashima R, Mochida S, Matsui A, YouLuTuZ Y, Ishikawa K, Toshima K, et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun 1999; 256: 527–31.
Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE . Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci 2007; 96: 2–15.
Yang H, Guo H, Fan K, Zhang B, Zhao L, Hou S, et al. Clearance of Propionibacterium acnes by kupffer cells is regulated by osteopontin through modulating the expression of p47phox. Mol Immunol 2011; 48: 2019–26.
Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H . Mechanisms of immune-mediated liver injury. Toxicol Sci 2010; 115: 307–21.
Welch KD, Reilly TP, Bourdi M, Hays T, Pise-Masison CA, Radonovich MF, et al. Genomic identification of potential risk factors during acetaminophen-induced liver disease in susceptible and resistant strains of mice. Chem Res Toxicol 2006; 19: 223–33.
Hinson JA, Roberts DW, James LP . Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 2010; (196): 369–405.
Laskin DL, Gardner CR, Price VF, Jollow DJ . Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology 1995; 21: 1045–50.
Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA . Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999; 30: 186–95.
Jaeschke H . Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 2005; 1: 389–97.
Ramaiah SK, Rittling S . Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci 2008; 103: 4–13.
Rittling SR . Osteopontin in macrophage function. Exp Rev Mol Med 2011; 13: e15.
Liu ZX, Han D, Gunawan B, Kaplowitz N . Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006; 43: 1220–30.
Fan K, Dai J, Wang H, Wei H, Cao Z, Hou S, et al. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum 2008; 58: 2041–52.
Chen Q, Kon J, Ooe H, Sasaki K, Mitaka T . Selective proliferation of rat hepatocyte progenitor cells in serum-free culture. Nat Protoc 2007; 2: 1197–205.
Holt MP, Cheng L, Ju C . Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol 2008; 84: 1410–21.
Han D, Shinohara M, Ybanez MD, Saberi B, Kaplowitz N . Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol 2010; (196): 267–310.
Martin-Murphy BV, Holt MP, Ju C . The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 2010; 192: 387–94.
Fan K, Zhang B, Yang H, Wang H, Tan M, Hou S, et al. A humanized anti-osteopontin antibody protects from Concanavalin A induced-liver injury in mice. Eur J Pharmacol 2011; 657: 144–51.
Sahai A, Pan X, Paul R, Malladi P, Kohli R, Whitington PF . Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am J Physiol Gastrointest Liver Physiol 2006; 291: G55–62.
Atul Sahai PM, Melin-Aldana H, Green RM, Whitington PF . Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol 2004; 287: G264–73.
Cantor H, Shinohara ML . Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev 2009; 9: 137–41.
Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H . Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 2002; 67: 322–8.
Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, et al. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther 2012; 340: 134–42.
Michael SL, Mayeux PR, Bucci TJ, Warbritton AR, Irwin LK, Pumford NR, et al. Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric Oxide 2001; 5: 432–41.
Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 2008; 14: 156–65.
Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, Mukaida N . The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol 2004; 75: 59–67.
Schwabe RF, Brenner DA . Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 2006; 290: G583–9.
Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, et al. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol 2003; 192: 119–30.
Ishibe T, Kimura A, Ishida Y, Takayasu T, Hayashi T, Tsuneyama K, et al. Reduced acetaminophen-induced liver injury in mice by genetic disruption of IL-1 receptor antagonist. Lab Invest 2009; 89: 68–79.
Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI . Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 1996; 24: 181–9.
James LP, Lamps LW, McCullough S, Hinson JA . Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun 2003; 309: 857–63.
Ramaiah SK, Jaeschke H . Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol 2007; 35: 757–66.
Koh A, da Silva AP, Bansal AK, Bansal M, Sun C, Lee H, et al. Role of osteopontin in neutrophil function. Immunology 2007; 122: 466–75.
Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES . Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem 2007; 282: 19463–72.
Kazanecki CC, Uzwiak DJ, Denhardt DT . Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem 2007; 102: 912–24.
Kim HJ, Lee HJ, Jun JI, Oh Y, Choi SG, Kim H, et al. Intracellular cleavage of osteopontin by caspase-8 modulates hypoxia/reoxygenation cell death through p53. Proc Natl Acad Sci U S A 2009; 106: 15326–31.
Acknowledgements
This work is supported in part by grants from Ministry of Science and Technology of China (2010CB945600, 2011CB966200), National Natural Science Foundation of China, the Special Project for Infection Disease (2008ZX10002-019), New Drug Development and Program of Shanghai Subject Chief Scientists (10XD1405400).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, Cy., Liang, Bb., Fan, Xy. et al. The dual role of osteopontin in acetaminophen hepatotoxicity. Acta Pharmacol Sin 33, 1004–1012 (2012). https://doi.org/10.1038/aps.2012.47
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.47
Keywords
This article is cited by
-
Optimization of clofibrate with natural product sesamol for reducing liver injury induced by acetaminophen
Medicinal Chemistry Research (2023)
-
Metabolic modulation of acetaminophen-induced hepatotoxicity by osteopontin
Cellular & Molecular Immunology (2019)
-
Sasa veitchii extracts suppress acetaminophen-induced hepatotoxicity in mice
Environmental Health and Preventive Medicine (2017)