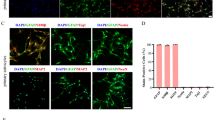
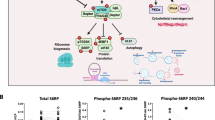
Abstract
Aim:
To investigate whether atorvastatin can promote formation of neurites in cultured cortical neurons and the signaling mechanisms responsible for this effect.
Methods:
Cultured rat cerebral cortical neurons were incubated with atorvastatin (0.05–10 μmol/L) for various lengths of time. For pharmacological experiments, inhibitors were added 30 min prior to addition of atorvastatin. Control cultures received a similar amount of DMSO. Following the treatment period, phase-contrast digital images were taken. Digital images of neurons were analyzed for total neurite branch length (TNBL), neurite number, terminal branch number, and soma area by SPOT Advanced Imaging software. After incubation with atorvastatin for 48 h, the levels of phosphorylated 3-phosphoinoside-dependent protein kinase-1 (PDK1), phospho-Akt, phosphorylated mammalian target of rapamycin (mTOR), phosphorylated 4E-binding protein 1 (4E-BP1), p70S6 kinase (p70S6K), and glycogen synthase kinase-3β (GSK-3β) in the cortical neurons were evaluated using Western blotting analyses.
Results:
Atorvastatin (0.05–10 μmol/L) resulted in dose-dependent increase in neurite number and length in these neurons. Pretreatment of the cortical neurons with phosphatidylinositol 3-kinase (PI3K) inhibitors LY294002 (30 μmol/L) and wortmannin (5 μmol/L), Akt inhibitor tricribine (1 μmol/L) or mTOR inhibitor rapamycin (100 nmol/L) blocked the atorvastatin-induced increase in neurite outgrowth, suggesting that atorvastatin promoted neurite outgrowth via activating the PI3K/Akt/mTOR signaling pathway. Atorvastatin (10 μmol/L) significantly increased the levels of phosphorylated PDK1, Akt and mTOR in the cortical neurons, which were prevented by LY294002 (30 μmol/L). Moreover, atorvastatin (10 μmol/L) stimulated the phosphorylation of 4E-BP1 and p70S6K, the substrates of mTOR, in the cortical neurons. In addition, atorvastatin (10 μmol/L) significantly increased the phosphorylated GSK-3β level in the cortical neurons, which was prevented by both LY294002 and tricribine.
Conclusion:
These results suggest that activation of both the PI3K/Akt/mTOR and Akt/GSK-3β signaling pathways is responsible for the atorvastatin-induced neurite outgrowth in cultured cortical neurons.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Asahi M, Huang Z, Thomas S, Yoshimura S, Sumii T, Mori T, et al. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab 2005; 25: 722–9.
Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, et al. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem 2005; 92: 1386–98.
Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma 2008; 25: 130–9.
Mahmood A, Goussev A, Kazmi H, Qu C, Lu D, Chopp M . Long-term benefits after treatment of traumatic brain injury with simvastatin in rats. Neurosurgery 2009; 65: 187–91.
Han X, Yang N, Xu Y, Zhu J, Chen Z, Liu Z, et al. Simvastatin treatment improves functional recovery after experimental spinal cord injury by upregulating the expression of BDNF and GDNF. Neurosci Lett 2011; 487: 255–9.
Fonseca AC, Resende R, Oliveira CR, Pereira CM . Cholesterol and statins in Alzheimer's disease: current controversies. Exp Neurol 2010; 223: 282–93.
Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, et al. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A 2001; 98: 5856–61.
Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S . Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med 2005; 2: e18.
Gellermann GP, Ullrich K, Tannert A, Unger C, Habicht G, Sauter SR, et al. Alzheimer-like plaque formation by human macrophages is reduced by fibrillation inhibitors and lovastatin. J Mol Biol 2006; 360: 251–7.
Won JS, Im YB, Khan M, Contreras M, Singh AK, Singh I . Lovastatin inhibits amyloid precursor protein (APP) beta-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts. J Neurochem 2008; 105: 1536–49.
Fernandez-Hernando C, Suarez Y, Lasuncion MA . Lovastatin-induced PC-12 cell differentiation is associated with RhoA/RhoA kinase pathway inactivation. Mol Cell Neurosci 2005; 29: 591–602.
Pooler AM, Xi SC, Wurtman RJ . The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J Neurochem 2006; 97: 716–23.
Holmberg E, Nordstrom T, Gross M, Kluge B, Zhang SX, Doolen S . Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma 2006; 23: 1366–78.
Evangelopoulos ME, Weis J, Kruttgen A . Mevastatin-induced neurite outgrowth of neuroblastoma cells via activation of EGFR. J Neurosci Res 2009; 87: 2138–44.
Read DE, Gorman AM . Involvement of Akt in neurite outgrowth. Cell Mol Life Sci 2009; 66: 2975–84.
Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 2003; 53: 743–51.
Kretz A, Schmeer C, Tausch S, Isenmann S . Simvastatin promotes heat shock protein 27 expression and Akt activation in the rat retina and protects axotomized retinal ganglion cells in vivo. Neurobiol Dis 2006; 21: 421–30.
Skaletz-Rorowski A, Lutchman M, Kureishi Y, Lefer DJ, Faust JR, Walsh K . HMG-CoA reductase inhibitors promote cholesterol-dependent Akt/PKB translocation to membrane domains in endothelial cells. Cardiovasc Res 2003; 57: 253–64.
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996; 15: 6541–51.
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM . RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A 1998; 95: 1432–7.
Brunn GJ, Fadden P, Haystead TA, Lawrence JC Jr . The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem 1997; 272: 32547–50.
Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G . Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 1997; 16: 3693–704.
Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr . Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci U S A 1998; 95: 7772–7.
Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M . Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci 2005; 25: 11300–12.
Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY . Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 2005; 25: 11288–99.
Dufner A, Thomas G . Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 1999; 253: 100–9.
Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 2001; 15: 2852–64.
Grimes CA, Jope RS . The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 2001; 65: 391–426.
Fan QW, Yu W, Gong JS, Zou K, Sawamura N, Senda T, et al. Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem 2002; 80: 178–90.
Schulz JG, Bosel J, Stoeckel M, Megow D, Dirnagl U, Endres M . HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J Neurochem 2004; 89: 24–32.
Van Aelst L, Cline HT . Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol 2004; 14: 297–304.
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J Clin Invest 2001; 108: 391–7.
Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 2000; 6: 1004–10.
Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994; 370: 527–32.
Ghosh-Choudhury N, Mandal CC, Choudhury GG . Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem 2007; 282: 4983–93.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008; 322: 963–6.
Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V . Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem 2010; 285: 28034–43.
Jaworski J, Sheng M . The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 2006; 34: 205–19.
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC . Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 2002; 10: 151–62.
Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 2003; 11: 1457–66.
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J . Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 2003; 13: 1259–68.
Hresko RC, Mueckler M . mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 2005; 280: 40406–16.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307: 1098–101.
Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N . Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J 1996; 15: 658–64.
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM . A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A 2002; 99: 467–72.
Schmelzle T, Hall MN . TOR, a central controller of cell growth. Cell 2000; 103: 253–62.
Herbert TP, Tee AR, Proud CG . The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem 2002; 277: 11591–6.
Hay N, Sonenberg N . Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–45.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–9.
Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS . A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells 2004; 18: 353–9.
Ooms LM, Fedele CG, Astle MV, Ivetac I, Cheung V, Pearson RB, et al. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell 2006; 17: 607–22.
Segarra J, Balenci L, Drenth T, Maina F, Lamballe F . Combined signaling through ERK, PI3K/AKT, and RAC1/p38 is required for met-triggered cortical neuron migration. J Biol Chem 2006; 281: 4771–8.
Goold RG, Owen R, Gordon-Weeks PR . Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci 1999; 112: 3373–84.
Acknowledgements
This work was supported by grant from Education Commission of Liaoning Province (LT2010064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, Y., Sui, Hj., Dong, Y. et al. Atorvastatin enhances neurite outgrowth in cortical neurons in vitro via up-regulating the Akt/mTOR and Akt/GSK-3β signaling pathways. Acta Pharmacol Sin 33, 861–872 (2012). https://doi.org/10.1038/aps.2012.59
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.59
Keywords
This article is cited by
-
Taurine ameliorates axonal damage in sciatic nerve of diabetic rats and high glucose exposed DRG neuron by PI3K/Akt/mTOR-dependent pathway
Amino Acids (2021)
-
Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice
Cell Death & Disease (2020)
-
Identification of an individual with a SYNGAP1 pathogenic mutation in India
Molecular Biology Reports (2020)
-
Transcriptional and Epigenetic Regulation in Injury-Mediated Neuronal Dendritic Plasticity
Neuroscience Bulletin (2017)
-
Neuronal cholesterol metabolism increases dendritic outgrowth and synaptic markers via a concerted action of GGTase-I and Trk
Scientific Reports (2016)