Abstract
Aim:
To investigate whether luteolin, a highly prevalent flavonoid, reverses the effects of epithelial-mesenchymal transition (EMT) in vitro and in vivo and to determine the mechanisms underlying this reversal.
Methods:
Murine malignant melanoma B16F10 cells were exposed to 1% O2 for 24 h. Cellular mobility and adhesion were assessed using Boyden chamber transwell assay and cell adhesion assay, respectively. EMT-related proteins, such as E-cadherin and N-cadherin, were examined using Western blotting. Female C57BL/6 mice (6 to 8 weeks old) were injected with B16F10 cells (1×106 cells in 0.2 mL per mouse) via the lateral tail vein. The mice were treated with luteolin (10 or 20 mg/kg, ip) daily for 23 d. On the 23rd day after tumor injection, the mice were sacrificed, and the lungs were collected, and metastatic foci in the lung surfaces were photographed. Tissue sections were analyzed with immunohistochemistry and HE staining.
Results:
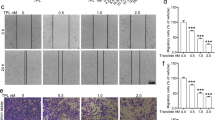
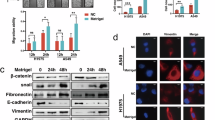
Hypoxia changed the morphology of B16F10 cells in vitro from the cobblestone-like to mesenchymal-like strips, which was accompanied by increased cellular adhesion and invasion. Luteolin (5−50 μmol/L) suppressed the hypoxia-induced changes in the cells in a dose-dependent manner. Hypoxia significantly decreased the expression of E-cadherin while increased the expression of N-cadherin in the cells (indicating the occurrence of EMT-like transformation), which was reversed by luteolin (5 μmol/L). In B16F10 cells, luteolin up-regulated E-cadherin at least partly via inhibiting the β3 integrin/FAK signal pathway. In experimental metastasis model mice, treatment with luteolin (10 or 20 mg/kg) reduced metastatic colonization in the lungs by 50%. Furthermore, the treatment increased the expression of E-cadherin while reduced the expression of vimentin and β3 integrin in the tumor tissues.
Conclusion:
Luteolin inhibits the hypoxia-induced EMT in malignant melanoma cells both in vitro and in vivo via the regulation of β3 integrin, suggesting that luteolin may be applied as a potential anticancer chemopreventative and chemotherapeutic agent.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hutchinson L . Skin cancer: Novel resistance mechanism revealed. Nat Rev Clin Oncol 2012; 9: 5.
Berk DR, LaBuz E, Dadras SS, Johnson DL, Swetter SM . Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults — the Stanford experience 1995–2008. Pediatr Dermatol 2010; 27: 244–54.
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology 2007; 114: 2309–15.
Weir HK, Marrett LD, Cokkinides V, Barnholtz-Sloan J, Patel P, Tai E, et al. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J Am Acad Dermatol 2011; 65: S38–49.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–15.
Dave B, Mittal V, Tan NM, Chang JC . Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res 2012; 14: 202.
Kushiro K, Chu RA, Verma A, Nunez NP . Adipocytes promote B16BL6 melanoma cell invasion and the epithelial-to-mesenchymal transition. Cancer Microenviron 2012; 5: 73–82.
Sun H, Hu K, Wu M, Xiong J, Yuan L, Tang Y, et al. Contact by melanoma cells causes malignant transformation of human epithelial-like stem cells via alpha V integrin activation of transforming growth factor beta1 signaling. Exp Biol Med (Maywood) 2011; 236: 352–65.
Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9: e1001162.
Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B . The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-kappaB/Snail/RKIP/PTEN circuit. Genes Cancer 2010; 1: 409–20.
Alonso SR, Tracey L, Ortiz P, Perez-Gomez B, Palacios J, Pollan M, et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res 2007; 67: 3450–60.
Lin Y, Shi R, Wang X, Shen HM . Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008; 8: 634–46.
Zhou Q, Yan B, Hu X, Li XB, Zhang J, Fang J . Luteolin inhibits invasion of prostate cancer PC3 cells through E-cadherin. Mol Cancer Ther 2009; 8: 1684–91.
Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL . Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem 2009; 284: 22825–33.
Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao M, et al. Ligustrazine inhibits B16F10 melanoma metastasis and suppresses angiogenesis induced by vascular endothelial growth factor. Biochem Biophys Res Commun 2009; 386: 374–9.
Chen W, Lu Y, Wu J, Gao M, Wang A, Xu B . Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother Pharmacol 2011; 67: 799–808.
Zhang LJ, Chen L, Lu Y, Wu JM, Xu B, Sun ZG, et al. Danshensu has anti-tumor activity in B16F10 melanoma by inhibiting angiogenesis and tumor cell invasion. Eur J Pharmacol 2010; 643: 195–201.
Jiang J, Tang YL, Liang XH . EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther 2011; 11: 714–23.
Jing Y, Han Z, Zhang S, Liu Y, Wei L . Epithelial-mesenchymal transition in tumor microenvironment. Cell Biosci 2011; 1: 29.
Hakomori SI . Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS Lett 2010; 584: 1901–6.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148: 349–61.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–15.
Nakashima S, Matsuda H, Oda Y, Nakamura S, Xu F, Yoshikawa M . Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg Med Chem 2010; 18: 2337–45.
Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res 2006; 66: 4826–34.
Byun S, Lee KW, Jung SK, Lee EJ, Hwang MK, Lim SH, et al. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res 2010; 70: 2415–23.
Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, et al. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med 2011; 50: 1599–609.
Zhou Q, Yan B, Hu X, Li XB, Zhang J, Fang J . Luteolin inhibits invasion of prostate cancer PC3 cells through E-cadherin. Mol Cancer Ther 2009; 8: 1684–91.
Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci 2011; 102: 1829–39.
Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei J, et al. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol 2008; 19: 380–7.
Sumual S, Saad S, Tang O, Yong R, McGinn S, Chen XM, et al. Differential regulation of Snail by hypoxia and hyperglycemia in human proximal tubule cells. Int J Biochem Cell Biol 2010; 42: 1689–97.
van MH, Verslype C, Windmolders P, van ER, Nevens F, van PJ . Characterization of a cell culture model for clinically aggressive hepatocellular carcinoma induced by chronic hypoxia. Cancer Lett 2012; 315: 178–88.
Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, et al. E-cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol 2011; 99: 392–7.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–90.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148: 349–61.
Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011; 146: 148–63.
Qian F, Vaux DL, Weissman IL . Expression of the integrin alpha 4 beta 1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell 1994; 77: 335–47.
Selivanova G, Ivaska J . Integrins and mutant p53 on the road to metastasis. Cell 2009; 139: 1220–2.
Acknowledgements
This project was supported in part by the National Natural Science Foundation of China (No 81173174), the National Key Technologies R & D Program of China during the 11th Five-Year Plan Period (No 2008BAI51B02), the PhD Programs Foundation of Ministry of Education of China (No 20113237110008), the Natural Science Foundation of Jiangsu Province (Nos BK2010085 and 2010562), Jiangsu Provincial Projects of International Cooperation and Exchanges of Jiangsu Province I (SBZ200900175) Educational Commission of Jiangsu Province (No 09KJA360002), Six Talents Peak Topics in Jiangsu Province (08-A-012), Open Project of Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica (P09013), Jiangsu College Graduate Research and Innovation Projects (2012-622 and CXLX11_0771), and a project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruan, Js., Liu, Yp., Zhang, L. et al. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin 33, 1325–1331 (2012). https://doi.org/10.1038/aps.2012.93
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2012.93
Keywords
This article is cited by
-
Stachytarpheta cayennensis-mediated copper nanoparticles shows anticancer activity in both in vitro and in vivo models
Applied Nanoscience (2023)
-
Dietary compounds and cutaneous malignant melanoma: recent advances from a biological perspective
Nutrition & Metabolism (2019)
-
Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87
Environmental Science and Pollution Research (2018)
-
Inhibiting STAT3 signaling is involved in the anti-melanoma effects of a herbal formula comprising Sophorae Flos and Lonicerae Japonicae Flos
Scientific Reports (2017)