Abstract
Aim:
(±)Doxazosin is a long-lasting inhibitor of α1-adrenoceptors that is widely used to treat benign prostatic hyperplasia and lower urinary tract symptoms. In this study we investigated the stereoselective binding of doxazosin enantiomers to the plasma proteins of rats, dogs and humans in vitro.
Methods:
Human, dog and rat plasma were prepared. Equilibrium dialysis was used to determine the plasma protein binding of each enantiomer in vitro. Chiral HPLC with fluorescence detection was used to measure the drug concentrations on each side of the dialysis membrane bag.
Results:
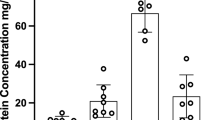
Both the enantiomers were highly bound to the plasma proteins of rats, dogs and humans [(−)doxazosin: 89.4%–94.3%; (+)doxazosin: 90.9%–95.4%]. (+)Doxazosin exhibited significantly higher protein binding capacities than (−)doxazosin in all the three species, and the difference in the bound concentration (Cb) between the two enantiomers was enhanced as their concentrations were increased. Although the percentage of the plasma protein binding in the dog plasma was significantly lower than that in the human plasma at 400 and 800 ng/mL, the corrected percentage of plasma protein binding was dog>human>rat.
Conclusion:
(−)Doxazosin and (+)doxazosin show stereoselective plasma protein binding with a significant species difference among rats, dogs and humans.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chapple CR . Selective alpha 1-adrenoceptor antagonists in benign prostatic hyperplasia: rationale and clinical experience. Eur Urol 1996; 29: 129–44.
Kirby RS, Andersen M, Gratzke P, Dahlstrand C, Høye K . A combined analysis of double-blind trials of the efficacy and tolerability of doxazosin-gastrointestinal therapeutic system, doxazosin standard and placebo in patients with benign prostatic hyperplasia. BJU Int 2001; 87: 192–200.
Montorsi F, Moncada I . Safety and tolerability of treatment for BPH. Eur Urol Suppl 2006; 5: 1004–12.
Barendrecht MM, Koopmans RP, De La Rosette JJMCH, Michel MC . Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: the cardiovascular system. BJU Int 2005; 95: 19–28.
ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat). JAMA 2000; 283: 1967–75.
Zhao D, Duan LH, Wang FY, Wang M, Lu HG, Wu ZG, et al. Chiral recognition of doxazosin enantiomers in 3 targets for therapy as well as adverse drug reactions in animal experiments. Can J Physiol Pharmacol 2012; 90: 1623–33.
Williams K . Molecular asymmetry and its pharmacological consequences. Adv Pharmacol 1991; 22: 57–135.
Pacifici GM, Viani A . Methods of determining plasma and tissue binding of drugs. Pharmacokinetic consequences. Clin Pharmacokinet 1992; 23: 449–68.
Hong Y, Tang Y, Zeng S . Enantioselective plasma protein binding of propafenone: Mechanism, drug interaction, and species difference. Chirality 2009; 21: 692–8.
Sun DL, Huang SD, Wu PS, Li J, Ye YJ, Jiang HD . Stereoselective protein binding of tetrahydropalmatine enantiomers in human plasma, HSA, and AGP, but not in rat plasma. Chirality 2010; 22: 618–23.
Maddi S, Yamsani MR, Seeling A, Scriba GKE . Stereoselective plasma protein binding of amlodipine. Chirality 2010; 22: 262–6.
Tang YH, Wang JY, Hu HH, Yao TW, Zeng S . Analysis of species-dependent hydrolysis and protein binding of esmolol enantiomers. J Pharm Anal 2012; 2: 220–5.
Howard ML, Hill JJ, Galluppi GR, McLean MA . Plasma protein binding in drug discovery and development. Comb Chem High Throughput Screen 2010; 13: 170–87.
Barre J, Chamouard JM, Houin G, Tillement JP . Equilibrium dialysis, ultrafiltration, and ultracentrifugation compared for determining the plasma-protein-binding characteristics of valproic acid. Clin Chem 1985; 31: 60–4.
Mohamed N, Kuroda Y, Shibukawa A, Nakagawa T, El Gizawy S, Askal H, et al. Enantioselective binding analysis of verapamil to plasma lipoproteins by capillary electrophoresis-frontal analysis. J Chromatogr A 2000; 875: 447–53.
Kaye B, Cussans N, Faulkner J, Stopher D, Reid J . The metabolism and kinetics of doxazosin in man, mouse, rat and dog. Br J Clin Pharmacol 1986; 21 Suppl 1: 19S–25S.
Gewirtz DA, Holt SA . Protein binding as a component of drug interaction in cellular pharmacokinetic studies: Effects of probenecid on transport and accumulation of methotrexate in Ehrlich ascites tumor cells in vitro. Biochem Pharmacol 1985; 34: 747–54.
Yü TF, Dayton PG, Gutman AB . Mutual suppression of the uricosuric effects of sulfinpyrazone and salicylate: a study in interactions between drugs. J Clin Invest 1963; 42: 1330–9.
Young R, Brogden R . Doxazosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in mild or moderate hypertension. Drugs 1988; 35: 525–41.
Liu K, Zhong D, Chen X . Enantioselective determination of doxazosin in human plasma by liquid chromatography-tandem mass spectrometry using ovomucoid chiral stationary phase. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2415–20.
Mizushima H, Takanaka K, Abe K, Fukazawa I, Ishizuka H . Stereoselective pharmacokinetics of oxybutynin and N-desethyloxybutynin in vitro and in vivo. Xenobiotica 2007; 37: 59–73.
Brocks DR . Drug disposition in three dimensions: an update on stereoselectivity in pharmacokinetics. Biopharm Drug Dispos 2006; 27: 387–406.
Acknowledgements
This work was supported by grants from the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” of China (No 2011ZX 09102-011-04) and the National Program on Key Basic Research Project of China (973 Program) (No 2012CB518601).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Ja., Kong, Dz., Zhen, Yq. et al. Stereoselective binding of doxazosin enantiomers to plasma proteins from rats, dogs and humans in vitro. Acta Pharmacol Sin 34, 1568–1574 (2013). https://doi.org/10.1038/aps.2013.120
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.120