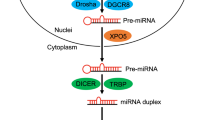
Abstract
MicroRNAs (miRNAs) are small non-coding RNA molecules, whose primary function is to regulate gene expression at the post-transcriptional/translational levels. MiRNAs play crucial roles in normal biological processes and are commonly dys-regulated in human diseases. Stem cells are regarded as the “mother” cells of all types of differentiated cells that comprise tissues and organs of the body. A novel hypothesis proposes that tumors are composed of heterogeneous cells derived from cancer stem cells, which have self-renewal and differentiation capabilities similar to those of normal stem cells. Cancer stem cells have been isolated and characterized from various tumors. Given recent studies supporting the critical regulatory roles of miRNAs in the self-renewal and differentiation of cancer stem cells, better understanding the functions of miRNAs will provide invaluable insights into the prevention of tumorigenesis and tumor progression. In this review, we will summarize the research progress in the study of miRNAs involved in the self-renewal and differentiation of cancer stem cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Becker AJ, Mc CE, Till JE . Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963; 197: 452–4.
Thomson JA, Itskovitz–Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–7.
Wicha MS, Liu S, Dontu G . Cancer stem cells: an old idea — a paradigm shift. Cancer Res 2006; 66: 1883–90.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–7.
Visvader JE, Lindeman GJ . Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8: 755–68.
Ambros V . microRNAs: tiny regulators with great potential. Cell 2001; 107: 823–6.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–97.
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23: 4051–60.
Borchert GM, Lanier W, Davidson BL . RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 2006; 13: 1097–101.
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425: 415–9.
Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U . Nuclear export of microRNA precursors. Science 2004; 303: 95–8.
Bohnsack MT, Czaplinski K, Gorlich D . Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004; 10: 185–91.
Bernstein E, Caudy AA, Hammond SM, Hannon GJ . Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001; 409: 363–6.
Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C elegans developmental timing. Cell 2001; 106: 23–34.
Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD . A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001; 293: 834–8.
Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH . Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev 2001; 15: 2654–9.
Knight SW, Bass BL . A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 2001; 293: 2269–71.
Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, et al. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 2002; 16: 720–8.
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R . Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 2006; 20: 515–24.
Liu C, Tang DG . MicroRNA regulation of cancer stem cells. Cancer Res 2011; 71: 5950–4.
Xia MHP . Great potential of microRNA in cancer stem cell. J Cancer Mol 2008; 4: 79–89.
Sakariassen PO, Immervoll H, Chekenya M . Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 2007; 9: 882–92.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100: 3983–8.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–8.
O'Brien CA, Pollett A, Gallinger S, Dick JE . A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–10.
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67: 1030–7.
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature 2008; 451: 345–9.
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008; 13: 153–66.
Bednar F, Simeone DM . Pancreatic cancer stem cells and relevance to cancer treatments. J Cell Biochem 2009; 107: 40–5.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1: 555–67.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1: 313–23.
Son MJ, Woolard K, Nam DH, Lee J, Fine HA . SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 2009; 4: 440–52.
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 2007; 104: 10158–63.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445: 111–5.
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 2007; 104: 973–8.
Bapat SA, Mali AM, Koppikar CB, Kurrey NK . Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 2005; 65: 3025–9.
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005; 65: 9328–37.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ . Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65: 10946–51.
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007; 132: 2542–56.
Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K . Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009; 69: 7507–11.
Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 2010; 16: 2580–90.
Bu Y, Cao D . The origin of cancer stem cells. Front Biosci (Schol Ed) 2012; 4: 819–30.
Iliopoulos D, Hirsch HA, Wang G, Struhl K . Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A 2011; 108: 1397–402.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–70.
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 2003; 100: 15178–83.
Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 2005; 19: 489–501.
Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R . DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 2007; 39: 380–5.
Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I . MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008; 455: 1124–8.
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS . MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009; 137: 647–58.
Peter ME . Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 2009; 8: 843–52.
Li X, Zhang J, Gao L, McClellan S, Finan MA, Butler TW, et al. MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death Differ 2012; 19: 378–86.
Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008; 68: 7846–54.
Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol 2008; 28: 6426–38.
Peng C, Li N, Ng YK, Zhang J, Meier F, Theis FJ, et al. A unilateral negative feedback loop between miR-200 microRNAs and Sox2/E2F3 controls neural progenitor cell-cycle exit and differentiation. J Neurosci 2012; 32: 13292–308.
Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN . Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J 2009; 28: 3157–70.
Wang G, Guo X, Hong W, Liu Q, Wei T, Lu C, et al. Critical regulation of miR-200/ZEB2 pathway in Oct4/Sox2-induced mesenchymal-to-epithelial transition and induced pluripotent stem cell generation. Proc Natl Acad Sci U S A 2013; 110: 2858–63.
Melton C, Blelloch R . MicroRNA regulation of embryonic stem cell self-renewal and differentiation. Adv Exp Med Biol 2010; 695: 105–17.
Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S . LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005; 132: 885–96.
Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, et al. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 2010; 6: 323–35.
Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 2010; 28: 1060–70.
Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R . Embryonic stem cell-specific microRNAs regulate the G1–S transition and promote rapid proliferation. Nat Genet 2008; 40: 1478–83.
Melton C, Judson RL, Blelloch R . Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 2010; 463: 621–6.
Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res 2007; 67: 7713–22.
Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M . MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res 2008; 18: 549–57.
Deng XG, Qiu RL, Wu YH, Li ZX, Xie P, Zhang J, et al. Overexpression of miR-122 promotes the hepatic differentiation and maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive feedback loop. Liver Int 2013 Jul 5. doi: 10.1111/liv.12239.
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131: 1109–23.
Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009; 138: 592–603.
Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, et al. MIR-451 and imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun 2008; 376: 86–90.
Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 2009; 50: 472–80.
Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17: 211–5.
Zhang H, Li W, Nan F, Ren F, Wang H, Xu Y, et al. MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line. Biochem Biophys Res Commun 2011; 404: 273–8.
Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim SW, et al. MicroRNA profiling of a CD133(+) spheroid-forming subpopulation of the OVCAR3 human ovarian cancer cell line. BMC Med Genomics 2012; 5: 18.
Hagan JP, Piskounova E, Gregory RI . Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 2009; 16: 1021–5.
Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009; 138: 696–708.
Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K . Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell 2010; 39: 761–72.
Lim YY, Wright JA, Attema JL, Gregory PA, Bert AG, Smith E, et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci 2013; 126: 2256–66.
Acknowledgements
This study is supported by an American Cancer Society Research Scholar Grant (RSG-13-265-01-RMC, to Yaguang XI) and an NIH/NCI R21 Grant (5R21CA160280, to Yaguang XI). We sincerely apologize to those colleagues whose work have not be cited due to time and space constraints.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Wang, Zm., Du, Wj., Piazza, G. et al. MicroRNAs are involved in the self-renewal and differentiation of cancer stem cells. Acta Pharmacol Sin 34, 1374–1380 (2013). https://doi.org/10.1038/aps.2013.134
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.134
Keywords
This article is cited by
-
Induction of E6AP by microRNA-302c dysregulation inhibits TGF-β-dependent fibrogenesis in hepatic stellate cells
Scientific Reports (2020)
-
MiR-34 and MiR-200: Regulator of Cell Fate Plasticity and Neural Development
NeuroMolecular Medicine (2019)
-
MicroRNA-638 inhibits cell proliferation by targeting suppress PIM1 expression in human osteosarcoma
Tumor Biology (2016)
-
Tumor suppressor miR-1 restrains epithelial-mesenchymal transition and metastasis of colorectal carcinoma via the MAPK and PI3K/AKT pathway
Journal of Translational Medicine (2014)
-
miR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial–mesenchymal transition
Cell Death & Disease (2014)