Abstract
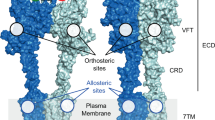
The task of finding selective and stable peptide receptor agonists with low molecular weight, desirable pharmacokinetic properties and penetrable to the blood-brain barrier has proven too difficult for many highly coveted drug targets, including receptors for endothelin, vasoactive intestinal peptide and galanin. These receptors and ligand-gated ion channels activated by structurally simple agonists such as glutamate, glycine and GABA present such a narrow chemical space that the design of subtype-selective molecules capable of distinguishing a dozen of glutamate and GABA receptor subtypes and possessing desirable pharmacokinetic properties has also been problematic. In contrast, the pharmaceutical industry demonstrates a remarkable success in developing 1,4-benzodiazepines, positive allosteric modulators (PMAs) of the GABAA receptor. They were synthesized over 50 years ago and discovered to have anxiolytic potential through an in vivo assay. As exemplified by Librium, Valium and Dormicum, these allosteric ligands of the receptor became the world's first blockbuster drugs. Through molecular manipulation over the past 2 decades, including mutations and knockouts of the endogenous ligands or their receptors, and by in-depth physiological and pharmacological studies, more peptide and glutamate receptors have become well-validated drug targets for which an agonist is sought. In such cases, the pursuit for PAMs has also intensified, and a working paradigm to identify drug candidates that are designed as PAMs has emerged. This review, which focuses on the general principles of finding PAMs of peptide receptors in the 21st century, describes the workflow and some of its resulting compounds such as PAMs of galanin receptor 2 that act as potent anticonvulsant agents.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Changeux JP . Allosteric proteins: from regulatory enzymes to receptors-personal recollections. Bioessays 1993; 15: 625–34.
Changeux JP . 50th anniversary of the word “allosteric”. Protein Sci 2011; 20: 1119–24.
Changeux JP . Allostery and the Monod-Wyman-Changeux model after 50 years. Annu Rev Biophys 2012; 41: 103–33.
Changeux JP, Gerhart JC, Schachman HK . Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry 1968; 7: 531–8.
Changeux JP, Rubin MM . Allosteric interactions in aspartate transcarbamylase. 3. Interpretation of experimental data in terms of the model of Monod, Wyman, and Changeux. Biochemistry 1968; 7: 553–61.
Monod J, Wyman J, Changeux JP . On the nature of allosteric transitions: a plausible model. J Mol Biol 1965; 12: 88–118.
Rubin MM, Changeux JP . On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol 1966; 21: 265–74.
Bellelli A, Brunori M . Hemoglobin allostery: variations on the theme. Biochim Biophys Acta 2011; 1807: 1262–72.
Fang TY, Zou M, Simplaceanu V, Ho NT, Ho C . Assessment of roles of surface histidyl residues in the molecular basis of the Bohr effect and of beta 143 histidine in the binding of 2,3-bisphosphoglycerate in human normal adult hemoglobin. Biochemistry 1999; 38: 13423–32.
Sternbach LH . The discovery of librium. Agents Actions 1972; 2: 193–6.
Sternbach LH . 1,4-benzodiazepines. Chemistry and some aspects of the structure-activity relationship. Angew Chem Int Ed Engl 1971; 10: 34–43.
Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, et al. Ivermectin: a positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 1998; 53: 283–94.
Urwyler S . Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev 2011; 63: 59–126.
Hoyer D, Bartfai T . Neuropeptides and neuropeptide receptors: drug targets, and peptide and non-peptide ligands: a tribute to professor Dieter Seebach. Chem Biodivers 2012; 9: 2367–87.
McColl CD, Jacoby AS, Shine J, Iismaa TP, Bekkers JM . Galanin receptor-1 knockout mice exhibit spontaneous epilepsy, abnormal EEGs and altered inhibition in the hippocampus. Neuropharmacology 2006; 50: 209–18.
Kenakin T, Miller LJ . Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 2010; 62: 265–304.
Kenakin T . Functional selectivity in GPCR modulator screening. Comb Chem High Throughput Screen 2008; 11: 337–43.
Govek SP, Bonnefous C, Hutchinson JH, Kamenecka T, McQuiston J, Pracitto R, et al. Benzazoles as allosteric potentiators of metabotropic glutamate receptor 2 (mGluR2): efficacy in an animal model for schizophrenia. Bioorg Med Chem Lett 2005; 15: 4068–72.
Kew JN . Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther 2004; 104: 233–44.
Kew JN, Kemp JA . Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005; 179: 4–29.
Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A 2001; 98: 13402–7.
Gregory KJ, Dong EN, Meiler J, Conn PJ . Allosteric modulation of metabotropic glutamate receptors: structural insights and therapeutic potential. Neuropharmacology 2011; 60: 66–81.
Marino MJ, Williams DL Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A 2003; 100: 13668–73.
Adams CL, Lawrence AJ . CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev 2007; 13: 308–16.
Shoichet BK, Kobilka BK . Structure–based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci 2012; 33: 268–72.
Rosenbaum DM, Rasmussen SG, Kobilka BK . The structure and function of G-protein-coupled receptors. Nature 2009; 459: 356–63.
Kirkpatrick A, Heo J, Abrol R, Goddard WA, 3rd . Predicted structure of agonist-bound glucagon-like peptide 1 receptor, a class B G protein-coupled receptor. Proc Natl Acad Sci U S A 2012; 109: 19988–93.
Lu X, Roberts E, Xia F, Sanchez-Alavez M, Liu T, Baldwin R, et al. GalR2-positive allosteric modulator exhibits anticonvulsant effects in animal models. Proc Natl Acad Sci U S A 2010; 107: 15229–34.
O'Donnell D, Ahmad S, Wahlestedt C, Walker P . Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol 1999; 409: 469–81.
Bloomquist BT, Beauchamp MR, Zhelnin L, Brown SE, Gore-Willse AR, Gregor P, et al. Cloning and expression of the human galanin receptor GalR2. Biochem Biophys Res Commun 1998; 243: 474–9.
Mitsukawa K, Lu X, Bartfai T . Galanin, galanin receptors, and drug targets. Exs 2010; 102: 7–23.
Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R . Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther 2006; 318: 700–8.
Lerner JT, Sankar R, Mazarati AM . Galanin and epilepsy. EXS 2010; 102: 183–94.
Kapur J . Galanin receptors modulate seizures. Epilepsy Curr 2011; 11: 125–7.
Land T, Langel U, Low M, Berthold M, Unden A, Bartfai T . Linear and cyclic N-terminal galanin fragments and analogs as ligands at the hypothalamic galanin receptor. Int J Pept Protein Res 1991; 38: 267–72.
Kask K, Berthold M, Kahl U, Nordvall G, Bartfai T . Delineation of the peptide binding site of the human galanin receptor. Embo J 1996; 15: 236–44.
Kask K, Berthold M, Kahl U, Jureus A, Nordvall G, Langel U, et al. Mutagenesis study on human galanin receptor GalR1 reveals domains involved in ligand binding. Ann NY Acad Sci 1998; 863: 78–85.
Fisone G, Berthold M, Bedecs K, Unden A, Bartfai T, Bertorelli R, et al. N-terminal galanin-(1–16) fragment is an agonist at the hippocampal galanin receptor. Proc Natl Acad Sci U S A 1989; 86: 9588–91.
Land T, Langel U, Bartfai T . Hypothalamic degradation of galanin (1–29) and galanin (1–16): identification and characterization of the peptidolytic products. Brain Res 1991; 558: 245–50.
Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A 2002; 99: 7136–41.
Ceide SC, Trembleau L, Haberhauer G, Somogyi L, Lu X, Bartfai T, et al. Synthesis of galmic: a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A 2004; 101: 16727–32.
Mazarati A, Lu X . Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides 2005; 39: 277–80.
Robertson CR, Pruess TH, Grussendorf E, White HS, Bulaj G . Generating orally active galanin analogues with analgesic activities. Chem Med Chem 2012; 7: 903–9.
Bulaj G, Green BR, Lee HK, Robertson CR, White K, Zhang L, et al. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem 2008; 51: 8038–47.
Parthiban M, Shanmughavel P . Three dimensional modeling of N-terminal region of galanin and its interaction with the galanin receptor. Bioinformation 2007; 2: 119–25.
Leger R, Thibaudeau K, Robitaille M, Quraishi O, van Wyk P, Bousquet-Gagnon N, et al. Identification of CJC-1131-albumin bioconjugate as a stable and bioactive GLP-1 (7–36) analog. Bioorg Med Chem Lett 2004; 14: 4395–8.
Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK . Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther 2010; 12: 790–7.
Acknowledgements
This manuscript is based on collaborations with Ulo LANGEL, Ed ROBERTS, Julius REBEK and Xiaoying LU. Several in vivo assays were carried out in other laboratories. We are most grateful to Claude WASTERLAIN, to the NINCDS for screening of anticonvulsants, and to Dale E Mais for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartfai, T., Wang, Mw. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharmacol Sin 34, 880–885 (2013). https://doi.org/10.1038/aps.2013.20
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.20
Keywords
This article is cited by
-
Co-released norepinephrine and galanin act on different timescales to promote stress-induced anxiety-like behavior
Neuropsychopharmacology (2021)
-
Old drugs with new skills: fenoprofen as an allosteric enhancer at melanocortin receptor 3
Cellular and Molecular Life Sciences (2017)
-
Homology modeling, docking, and molecular dynamics simulation of the receptor GALR2 and its interactions with galanin and a positive allosteric modulator
Journal of Molecular Modeling (2016)