Abstract
Aim:
NVP-BEZ235 is a novel dual PI3K/mTOR inhibitor and shows dramatic effects on gliomas. The aim of this study was to investigate the effects of NVP-BEZ235 on the radiosensitivity and autophagy of glioma stem cells (GSCs) in vitro.
Methods:
Human GSCs (SU-2) were tested. The cell viability and survival from ionizing radiation (IR) were evaluated using MTT and clonogenic survival assay, respectively. Immunofluorescence assays were used to identify the formation of autophagosomes. The apoptotic cells were quantified with annexin V-FITC/PI staining and flow cytometry, and observed using Hoechst 33258 staining and fluorescence microscope. Western blot analysis was used to analyze the expression levels of proteins. Cell cycle status was determined by measuring DNA content after staining with PI. DNA repair in the cells was assessed using a comet assay.
Results:
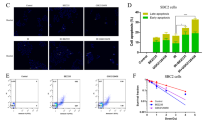
Treatment of SU-2 cells with NVP-BEZ235 (10–320 nmol/L) alone suppressed the cell growth in a concentration-dependent manner. A low concentration of NVP-BEZ235 (10 nmol/L) significantly increased the radiation sensitivity of SU-2 cells, which could be blocked by co-treatment with 3-MA (50 μmol/L). In NVP-BEZ235-treated SU-2 cells, more punctate patterns of microtubule-associated protein LC3 immunoreactivity was observed, and the level of membrane-bound LC3-II was significantly increased. A combination of IR with NVP-BEZ235 significantly increased the apoptosis of SU-2 cells, as shown in the increased levels of BID, Bax, and active caspase-3, and decreased level of Bcl-2. Furthermore, the combination of IR with NVP-BEZ235 led to G1 cell cycle arrest. Moreover, NVP-BEZ235 significantly attenuated the repair of IR-induced DNA damage as reflected by the tail length of the comet.
Conclusion:
NVP-BEZ235 increases the radiosensitivity of GSCs in vitro by activating autophagy that is associated with synergistic increase of apoptosis and cell-cycle arrest and decrease of DNA repair capacity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tunici P, Irvin D, Liu G, Yuan X, Zhaohui Z, Ng H, et al. Brain tumor stem cells: new targets for clinical treatments? Neurosurg Focus 2006; 20: E27.
Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol 2012; 109: 195–203.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Qiu B, Zhang D, Tao J, Wu A, Wang Y . A simplified and modified procedure to culture brain glioma stem cells from clinical specimens. Oncol Lett 2012; 3: 50–4.
Jamal M, Rath BH, Tsang PS, Camphausen K, Tofilon PJ . The brain microenvironment preferentially enhances the radioresistance of CD133 (+) glioblastoma stem-like cells. Neoplasia 2012; 14: 150–8.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–60.
Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochin Biophys Acta 2012; 1826: 338–49.
Wu J, Lai G, Wan F, Xiao Z, Zeng L, Wang X, et al. Knockdown of checkpoint kinase 1 is associated with the increased radiosensitivity of glioblastoma stem-like cells. Tohoku J Exp Med 2012; 226: 267–74.
Zhuang W, Li B, Long L, Chen L, Huang Q, Liang Z . Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int J Cancer 2011; 129: 2720–31.
Zhuang W, Li B, Long L, Chen L, Huang Q, Liang ZQ . Knockdown of the DNA-dependent protein kinase catalytic subunit radiosensitizes glioma-initiating cells by inducing autophagy. Brain Res 2011; 1371: 7–15.
Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 2008; 68: 8022–30.
Prevo R, Deutsch E, Sampson O, Diplexcito J, Cengel K, Harper J, et al. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res 2008; 68: 5915–23.
Gallia GL, Rand V, Siu IM, Eberhart CG, James CD, Marie SK, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res 2006; 4: 709–14.
Gomez-Pinillos A, Ferrari AC . mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol Oncol Clin North Am 2012; 26: 483–505.
Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther 2011; 10: 2426–36.
Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res 2012; 72: 239–48.
Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther 2009; 8: 2204–10.
Mukherjee B, Tomimatsu N, Amancherla K, Camacho CV, Pichamoorthy N, Burma S . The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia 2012; 14: 34–43.
Fokas E, Yoshimura M, Prevo R, Higgins G, Hackl W, Maira SM, et al. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol 2012; 7: 48.
Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res 2009; 69: 7644–52.
Sunayama J, Sato A, Matsuda K, Tachibana K, Suzuki K, Narita Y, et al. Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro Oncol 2010; 12: 1205–19.
Sunayama J, Matsuda K, Sato A, Tachibana K, Suzuki K, Narita Y, et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells 2010; 28: 1930–9.
Huang Q, Zhang QB, Dong J, Wu YY, Shen YT, Zhao YD, et al. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC Cancer 2008; 8: 304.
Chiarini F, Grimaldi C, Ricci F, Tazzari PL, Evangelisti C, Ognibene A, et al. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against T-cell acute lymphoblastic leukemia. Cancer Res 2010; 70: 8097–107.
Yang S, Xiao X, Meng X, Leslie KK . A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLoS One 2011; 6: e26343.
Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A 2012; 109: 2003–8.
Cerniglia GJ, Karar J, Tyagi S, Christofidou-Solomidou M, Rengan R, Koumenis C, et al. Inhibition of autophagy as a strategy to augment radiosensitization by the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Mol Pharmacol 2012; 82: 1230–40.
Kuma A, Matsui M, Mizushima N . LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy 2007; 3: 323–8.
Griffin C, McNulty J, Pandey S . Pancratistatin induces apoptosis and autophagy in metastatic prostate cancer cells. Int Oncol 2011; 38: 1549–56.
Zhang M, Jiang M, Bi Y, Zhu H, Zhou Z, Sha J . Autophagy and apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS One 2012; 7: e41412.
Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B . Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res 2009; 15: 6096–105.
Tai S, Sun Y, Liu N, Ding B, Hsia E, Bhuta S, et al. Combination of Rad001 (everolimus) and propachlor synergistically induces apoptosis through enhanced autophagy in prostate cancer cells. Mol Cancer Ther 2012; 11: 1320–31.
Frum R, Ramamoorthy M, Mohanraj L, Deb S, Deb SP . MDM2 controls the timely expression of cyclin A to regulate the cell cycle. Mol Cancer Res 2009; 7: 1253–67.
Fung TK, Ma HT, Poon RY . Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor. Mol Biol Cell 2007; 18: 1861–73.
Mori T, Ikeda DD, Fukushima T, Takenoshita S, Kochi H . NIRF constitutes a nodal point in the cell cycle network and is a candidate tumor suppressor. Cell Cycle 2011; 10: 3284–99.
Saha A, Halder S, Upadhyay SK, Lu J, Kumar P, Murakami M, et al. Epstein-Barr virus nuclear antigen 3C facilitates G1-S transition by stabilizing and enhancing the function of cyclin D1. PLoS Pathog 2011; 7: e1001275.
Mollah ML, Park DK, Park HJ . Cordyceps militaris grown on germinated soybean induces G2/M cell cycle arrest through downregulation of cyclin B1 and Cdc25c in human colon cancer HT-29 cells. Evid Based Complement Alternat Med 2012; 2012: 249217.
Jayakumar S, Bhilwade HN, Pandey BN, Sandur SK, Chaubey RC . The potential value of the neutral comet assay and the expression of genes associated with DNA damage in assessing the radiosensitivity of tumor cells. Mutat Res 2012; 748: 52–9.
Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao C, et al. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS One 2011; 6: e23427.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–95.
Ekman S, Wynes MW, Hirsch FR . The mTOR pathway in lung cancer and implications for therapy and biomarker analysis. J Thorac Oncol 2012; 7: 947–53.
Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A, et al. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clin Cancer Res 2010; 16: 5424–35.
Roper J, Richardson MP, Wang WV, Richard LG, Chen W, Coffee EM, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA wild-type colorectal cancer. PoLS One 2011; 6: e25132.
Elfiky AA, Aziz SA, Conrad PJ, Siddiqui S, Hackl W, Maira M, et al. Characterization and targeting of phosphatidylinositol-3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in renal cell cancer. J Transl Med 2011; 9: 133.
Civallero M, Cosenza M, Marcheselli L, Pozzi S, Sacchi S . NVP-BEZ235 alone and in combination in mantle cell lymphoma: an effective therapeutic strategy. Expert Opin Investig Drugs 2012; 21: 1597–606.
Hippert MM, O'Toole PS, Thorburn A . Autophagy in cancer: good, bad, or both? Cancer Res 2006; 66: 9349–51.
Zhuang W, Qin Z, Liang Z . The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim Biophys Sin (Shanghai) 2009; 41: 341–51.
Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev 2012; 133: 444–55.
Natsumeda M, Aoki H, Miyahara H, Yajima N, Uzuka T, Toyoshima Y, et al. Induction of autophagy in temozolomide treated malignant gliomas. Neuropathology 2011; 31: 486–93.
Altmeyer A, Josset E, Denis JM, Gueulette J, Slabbert J, Mutter D, et al. The mTOR inhibitor RAD001 augments radiation-induced growth inhibition in a hepatocellular carcinoma cell line by increasing autophagy. Int J Oncol 2012. doi: 10.3892/ijo.2012.1583.
Zhou S, Zhao L, Kuang M, Zhang B, Liang Z, Yi T, et al. Autophagy in tumorigenesis and cancer therapy: Dr Jekyll or Mr Hyde? Cancer Lett 2012; 323: 115–27.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos 30873052, 81072656, 81102466), Jiangsu Province's outstanding Medical Academic Leader program (No LJ201139) and Priority Academic Program Development of Jiangsu Higher Education Institutions, PAPD, Jiangsu Province's Key Medical Department in 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Wj., Long, Lm., Yang, N. et al. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol Sin 34, 681–690 (2013). https://doi.org/10.1038/aps.2013.22
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.22
Keywords
This article is cited by
-
The PI3K/AKT/mTOR signaling pathway inhibitors enhance radiosensitivity in cancer cell lines
Molecular Biology Reports (2021)
-
Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC
Journal of Experimental & Clinical Cancer Research (2019)
-
Enhanced efficacy of histone deacetylase inhibitor combined with bromodomain inhibitor in glioblastoma
Journal of Experimental & Clinical Cancer Research (2018)
-
NVP-BEZ235, a dual PI3K-mTOR inhibitor, suppresses the growth of FaDu hypopharyngeal squamous cell carcinoma and has a synergistic effect with Cisplatin
Cell Death Discovery (2018)
-
Influence of autophagy on the efficacy of radiotherapy
Radiation Oncology (2017)