Abstract
Aim:
To investigate the actions of the muscarinic agonist carbachol on glutamate-induced neurotoxicity in PC12 cells, and the underlying mechanisms.
Methods:
PC12 cells were treated with different concentrations of glutamate for 24 or 48 h. The cell viability was measured using MTT assay, and the expression and activation of GSK-3β were detected with Western blot. β-Catenin translocation was detected using immunofluorescence. Luciferase reporter assay and real-time PCR were used to analyze the transcriptional activity of β-catenin.
Results:
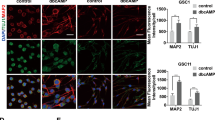
Glutamate (1, 3, and 10 mmol/L) induced PC12 cell death in a dose-dependent manner. Moreover, treatment of the cells with glutamate (1 mmol/L) caused significant overactivation of GSK-3β and prevented β-catenin translocation to the nucleus. Pretreatment with carbachol (0.01 μmol/L) blocked glutamate-induced cell death and GSK-3β overactivation, and markedly enhanced β-catenin transcriptional activity.
Conclusion:
Activation of muscarinic receptors exerts neuroprotection in PC12 cells by attenuating glutamate-induced GSK-3β overactivation, suggesting potential benefits of muscarinic agonists for Alzheimer's disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shah RS, Lee HG, Xiongwei Z, Perry G, Smith MA, Castellani RJ . Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother 2008; 62: 199–207.
Ladner CJ, Lee JM . Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J Neuropathol Exp Neurol 1998; 5: 719–31.
Fisher A . Therapeutic strategies in Alzheimer's disease: M1 muscarinic agonists. Jpn J Pharmacol 2000; 84: 101–12.
Longo FM, Massa SM . Neuroprotective strategies in Alzheimer's disease. NeuroRx 2004; 1: 117–27.
Caccamo A, Fisher A, LaFerla FM . M1 agonists as a potential disease-modifying therapy for Alzheimer's disease. Curr Alzheimer Res 2009; 6: 112–7.
Tobin AB, Budd DC . The anti-apoptotic response of the Gq/11-coupled muscarinic receptor family. Biochem Soc Trans 2003; 31: 1182–5.
Farias GG, Godoy JA, Hernandez F, Avila J, Fisher A, Inestrosa NC . M1 muscarinic receptor activation protects neurons from beta-amyloid toxicity. A role for Wnt signaling pathway. Neurobiol Dis 2004; 17: 337–48.
De Sarno P, Shestopal SA, King TD, Zmijewska A, Song L, Jope RS . Muscarinic receptor activation protects cells from apoptotic effects of DNA damage, oxidative stress, and mitochondrial inhibition. J Biol Chem 2003; 278: 11086–93.
Lindenboim L, Pinkas-Kramarski R, Sokolovsky M, Stein R . Activation of muscarinic receptors inhibits apoptosis in PC12M1 cells. J Neurochem 1995; 64: 2491–9.
Choi DW . Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988; 1: 623–34.
Olney JW . Excitotoxicity: an overview. Can Dis Wkly Rep 1990; 16: 47–57.
Doble A . The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 1999; 81: 163–221.
Dong XX, Wang Y, Qin ZH . Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 2009; 30: 379–87.
Lau A, Tymianski M . Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 2010; 460: 525–42.
Guan ZZ . Cross-talk between oxidative stress and modifications of cholinergic and glutaminergic receptors in the pathogenesis of Alzheimer's disease. Acta Pharmacol Sin 2008; 29: 773–80.
Dale TC . Signal transduction by the Wnt family of ligands. Biochem J 1998; 329: 209–23.
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P . Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 1996; 272: 1023–6.
Frame S, Cohen P . GSK3 takes centre stage more than 20 years after its discovery. Biochem J 2001; 359: 1–16.
Carmichael J, Sugars KL, Bao YP, Rubinsztein DC . Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem 2002; 277: 33791–8.
Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S . HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem 1999; 73: 578–86.
Pap M, Cooper GM . Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 1998; 273: 19929–32.
Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, et al. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci 2001; 13: 1913–22.
Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D . GSK-3beta: a bifunctional role in cell death pathways. Int J Cell Biol 2012; 2012: 930710.
Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S . Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3beta inhibition and in neurons. J Neural Transm 2000; 107: 1201–12.
del Ser T, Steinwachs KC, Gertz HJ, Andres MV, Gomez-Carrillo B, Medina M, et al. Treatment of Alzheimer's disease with the GSK-3 inhibitor tideglusib: a pilot study. J Alzheimers Dis 2013; 33: 205–15.
Fisher A . M1 muscarinic agonists target major hallmarks of Alzheimer's disease — an update. Curr Alzheimer Res 2007; 4: 577–80.
Martinez A, Alonso M, Castro A, Perez C, Moreno FJ . First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem 2002; 45: 1292–9.
Goode N, Hughes K, Woodgett JR, Parker PJ . Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem 1992; 267: 16878–82.
Shaw M, Cohen P, Alessi DR . Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett 1997; 416: 307–11.
Patapoutian A, Reichardt LF . Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol 2000; 10: 392–9.
Eldar-Finkelman H . Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 2002; 8: 126–32.
Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, et al. A novel GSK-3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis 2009; 35: 359–67.
Froissard P, Duval D . Cytotoxic effects of glutamic acid on PC12 cells. Neurochem Int 1994; 24: 485–93.
Lee JH, Lee EO, Kang JL, Chong YH . Concomitant degradation of beta-catenin and GSK-3 beta potently contributes to glutamate-induced neurotoxicity in rat hippocampal slice cultures. J Neurochem 2008; 106: 1066–77.
Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A 2000; 97: 11074–9.
Hetman M, Cavanaugh JE, Kimelman D, Xia Z . Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 2000; 20: 2567–74.
Crowder RJ, Freeman RS . Glycogen synthase kinase-3 beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J Biol Chem 2000; 275: 34266–71.
Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P . Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 1998; 8: 573–81.
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proc Natl Acad Sci U S A 2007; 104: 3591–6.
Giese KP . GSK-3: a key player in neurodegeneration and memory. IUBMB Life 2009; 61: 516–21.
Medina M, Avila J . Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimer's disease. Curr Pharm Des 2010; 16: 2790–8.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No 30873057 and 81171245) and the Key Basic Project of the Shanghai Municipal Science and Technology Commission, China (No 08JC1413600 and 11JC1406600).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, K., Yang, Lm., Chen, Hz. et al. Activation of muscarinic receptors inhibits glutamate-induced GSK-3β overactivation in PC12 cells. Acta Pharmacol Sin 34, 886–892 (2013). https://doi.org/10.1038/aps.2013.42
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.42