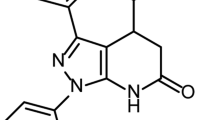
Abstract
Aim:
To investigate the effects of arbidol hydrochloride (ARB), a widely used antiviral agent, on the inflammation induced by influenza virus.
Methods:
MDCK cells were infected with seasonal influenza A/FM/1/47 (H1N1) or pandemic influenza A/Hubei/71/2009 (H1N1). In vitro cytotoxicity and antiviral activity of ARB was determined using MTT assay. BALB/c mice were infected with A/FM/1/47 (H1N1). Four hours later the mice were administered ARB (45, 90, and 180 mg·kg−1·d−1) or the neuraminidase inhibitor oseltamivir (22.5 mg·kg−1·d−1) via oral gavage once a day for 5 d. Body-weight, median survival time, viral titer, and lung index of the mice were measured. The levels of inflammatory cytokines were examined using real-time RT-PCR and ELISA.
Results:
Both H1N1 stains were equally sensitive to ARB as tested in vitro. In the infected mice, ARB (90 and 180 mg·kg−1·d−1) significantly decreased the mortality, alleviated virus-induced lung lesions and viral titers. Furthermore, ARB suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10 in the bronchoalveolar lavage fluids and lung tissues. However, ARB did not significantly affect the levels of IFN-α and IFN-γ, but reduced the level of IFN-β1 in lung tissues at 5 dpi. In peritoneal macrophages challenged with A/FM/1/47 (H1N1) or poly I:C, ARB (20 μmol/L) suppressed the levels of IL-1β, IL-6, IL-12, and TNF-α, and elevated the level of IL-10. Oseltamivir produced comparable alleviation of virus-induced lung lesions with more reduction in the viral titers, but less effective modulation of the inflammatory cytokines.
Conclusion:
ARB efficiently inhibits both H1N1 stains and diminishes both viral replication and acute inflammation through modulating the expression of inflammatory cytokines.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Clem A, Galwankar S . Seasonal influenza: waiting for the next pandemic. J Glob Infect Dis 2009; 1: 51–6.
Fields BN, Knipe DM, Howley PM . Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
La Gruta NL, Kedzierska K, Stambas J, Doherty PC . A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol 2007; 85: 85–92.
Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005; 310: 77–80.
CDC. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 433–5.
Cheng PK, Leung TW, Ho EC, Leung PC, Ng AY, Lai MY, et al. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1). Emerg Infect Dis 2009; 15: 966–8.
Zaraket H, Saito R, Suzuki Y, Baranovich T, Dapat C, Caperig-Dapat I, et al. Genetic makeup of amantadine-resistant and oseltamivir-resistant human influenza A/H1N1 viruses. J Clin Microbiol 2010; 48: 1085–92.
Ludwig S . Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J Antimicrob Chemother 2009; 64: 1–4.
Josset L, Textoris J, Loriod B, Ferraris O, Moules V, Lina B, et al. Gene expression signature-based screening identifies new broadly effective influenza A antivirals. PLoS One 2010; 5: e13169.
Leneva IA, Fediakina IT, Gus'kova TA, Glushkov RG . Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A. Ter Arkh 2005; 77: 84–8.
Leneva IA, Shuster AM . Antiviral etiotropic chemicals: efficacy against influenza A viruses a subtype H5N1. Vopr Virusol 2006; 51: 4–7.
Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol 2007; 152: 1447–55.
Leneva IA, Sokolova MV, Fediakina IT, Khristova ML, Fadeeva NI, Gus'kova TA . Study of the effect of antiviral drugs on the reproduction of the respiratory syncytial virus by enzyme immunoassay. Vopr Virusol 2002; 47: 42–5.
Zhong Q, Yang Z, Liu Y, Deng H, Xiao H, Shi L, et al. Antiviral activity of Arbidol against Coxsackie virus B5 in vitro and in vivo. Arch Virol 2009; 154: 601–7.
Deng HY, Luo F, Shi LQ, Zhong Q, Liu YJ, Yang ZQ . Efficacy of arbidol on lethal hantaan virus infections in suckling mice and in vitro. Acta Pharmacol Sin 2009; 30: 1015–24.
Chai H, Zhao Y, Zhao C, Gong P . Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem 2006; 14: 911–7.
Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ . Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 2008; 15: 997–1005.
Brooks MJ, Sasadeusz JJ, Tannock GA . Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr Opin Pulm Med 2004; 10: 197–203.
Teissier E, Zandomeneghi G, Loquet A, Lavillette D, Lavergne JP, Montserret R, et al. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One 2011; 6: e15874.
Leneva IA, Russell RJ, Boriskin YS, Hay AJ . Characteristics of arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol. Antivir Res 2009; 81: 132–40.
Brooks MJ, Burtseva EI, Ellery PJ, Marsh GA, Lew AM, Slepushkin AN, et al. Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions. J Med Virol 2012; 84: 170–81.
Glushkov RG, Gus'kova TA, Krylova L, Nikolaeva IS . Mechanisms of arbidole's immunomodulating action. Vestn Ross Akad Med Nauk 1999; (3): 36–40.
Silin DS, Lyubomska OV, Ershov FI, Frolov VM, Kutsyna GA . Synthetic and natural immunomodulators acting as interferon inducers. Curr Pharm Design 2009; 15: 1238–47.
Boriskin YS, Pecheur EI, Polyak SJ . Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J 2006; 3: 56.
Zhao JR, Li YD, Pan LM, Zhu N, Ni HX, Xu GZ, et al. Genetic characteristics of 2009 pandemic H1N1 influenza A viruses isolated from Mainland China. Virol Sin 2011; 26: 418–27.
Xu L, Bao L, Li F, Lv Q, Ma Y, Zhou J, et al. Adaption of seasonal H1N1 influenza virus in mice. PLoS One 2011; 6: e28901.
Drusano GL, Preston SL, Smee D, Bush K, Bailey K, Sidwell RW . Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing. Antimicrob Agents Chemother 2001; 45: 2115–8.
Triana-Baltzer GB, Gubareva LV, Nicholls JM, Pearce MB, Mishin VP, Belser JA, et al. Novel pandemic influenza A (H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One 2009; 4: e7788.
Yatmaz S, Seow HJ, Gualano RC, Wong ZX, Stambas J, Selemidis S, et al. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am J Respir Cell Mol Biol 2013; 48: 17–26.
De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest 2010; 120: 2395–405.
Zhang X, Goncalves R, Mosser DM . The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008; Chapter 14: Unit 14.1.
Prichard MN, Turk SR, Coleman LA, Engelhardt SL, Shipman C Jr, Drach JC . A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J Virol Methods 1990; 28: 101–6.
Leneva IA, Fadeeva NI, Fedyakina IT, Gus'kova TA, Khristova NL, Sokolova MV, et al. Use of enzyme immunoassay to identify virus-specific antigens in studying a new anti-influenza preparation, arbidol. Pharm Chem J 1994; 28: 506–605.
Glushkov RG, Fadeeva NI, Leneva IA, Gerasina SF, Budanova LI, Sokolova ND, et al. Molecular biological characteristics of the action of arbidol — A new antiviral drug. Pharm Chem J 1992; 26: 106–15.
Leneva IA, Fediakina IT, Eropkin M, Gudova NV, Romanovskaia AA, Danilenko DM, et al. Study of the antiviral activity of Russian anti-influenza agents in cell culture and animal models. Vopr Virusol 2010; 55: 19–27.
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, et al. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol 2007; 9: 1683–94.
Sladkova T, Kostolansky F . The role of cytokines in the immune response to influenza A virus infection. Acta Virol 2006; 50: 151–62.
Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC . Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol 2010; 84: 7569–80.
Pommerenke C, Wilk E, Srivastava B, Schulze A, Novoselova N, Geffers R, et al. Global transcriptome analysis in influenza-infected mouse lungs reveals the kinetics of innate and adaptive host immune responses. PLoS One 2012; 7: e41169.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 2002; 3: 196–200.
Murphy EA, Davis JM, McClellan JL, Carmichael MD, Rooijen NV, Gangemi JD . Susceptibility to infection and inflammatory response following influenza virus (H1N1, A/PR/8/34) challenge: role of macrophages. J Interferon Cytokine Res 2011; 31: 501–8.
Osterlund P, Pirhonen J, Ikonen N, Ronkko E, Strengell M, Makela SM, et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J Virol 2010; 84: 1414–22.
Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S . Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev 2001; 12: 171–80.
Nimmerjahn F, Dudziak D, Dirmeier U, Hobom G, Riedel A, Schlee M, et al. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J Gen Virol 2004; 85: 2347–56.
Matikainen S, Pirhonen J, Miettinen M, Lehtonen A, Govenius-Vintola C, Sareneva T, et al. Influenza A and sendai viruses induce differential chemokine gene expression and transcription factor activation in human macrophages. Virology 2000; 276: 138–47.
Acknowledgements
This work was supported by the National Mega Project on Major Drug Development (2009ZX09301-014-1), the National Natural Science Foundation of China (No 30873104 and 81000734) and the Fundamental Research Funds for the Central Universities (4101045).
We thank Prof Tian-xian LI for the viruses. We gratefully acknowledge all individuals within the ABSL-3 team for their kindness and their technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Xiong, Hr., Lu, L. et al. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin 34, 1075–1083 (2013). https://doi.org/10.1038/aps.2013.54
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.54
Keywords
This article is cited by
-
The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro
Cell Discovery (2020)
-
The Antiviral Drug Arbidol Inhibits Zika Virus
Scientific Reports (2018)
-
Identification of Inhibitory Compounds Against Singapore Grouper Iridovirus Infection by Cell Viability-Based Screening Assay and Droplet Digital PCR
Marine Biotechnology (2018)
-
The cytokine storm of severe influenza and development of immunomodulatory therapy
Cellular & Molecular Immunology (2016)
-
The Location of the Protonated and Unprotonated Forms of Arbidol in the Membrane: A Molecular Dynamics Study
The Journal of Membrane Biology (2016)