Abstract
Aim:
Retigeric acid B (RAB), a pentacyclic triterpenic acid from Lobaria kurokawae Yoshim, has been found to induce apoptosis in prostate cancer cells. The aim of this study was to investigate the roles of mitochondrial damage-caused mitophagy in RAB-induced prostate cancer cell death in vitro.
Methods:
Human prostate cancer PC3 and LNCaP cells were tested. Cell viability was analyzed with MTT assay. Cell apoptosis, ROS level and mitochondrial transmembrane potential (mtΔψ) were measured with flow cytometry. Autophagy- and apoptosis-related proteins were studied using Western blotting. GFP-LC3B puncta, mitochondrial swelling and mitophagy were examined morphologically. Quantitative RT-PCR was used to measure LC3B mRNA level, and siRNA was used to knock down LC3BII.
Results:
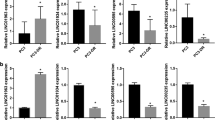
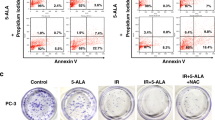
In both PC3 and LNCaP cells, RAB (15 μmol/L) increased ROS accumulation and decreased mtΔψ in a time-dependent manner. Furthermore, RAB induced mitochondrial swelling and mitophagy, significantly increased LC3B expression and conversion of LC3BI to LC3BII, and the elimination of mitochondria by LC3BII-containing autophagolysosomes. In addition, RAB suppressed the PI3K/Akt/mTOR pathway activation. Pretreatment of PC3 cells with autophagy inhibitor 3-MA (5 mmol/L) or the lysosomal protease inhibitor CQ (10 μmol/L) significantly increased RAB-induced apoptosis. Similar results were obtained in RAB-treated PC3 cells with LC3B knocked down.
Conclusion:
RAB induces mitochondrial damage and mitophagy that attenuates RAB-induced prostate cancer cell death. Thus, suppression of mitophagy might be a potential strategy for improving the chemotherapeutic effects of RAB.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mizushima N, Levine B, Cuervo AM, Klionsky DJ . Autophagy fights disease through cellular self-digestion. Nature 2008; 451: 1069–75.
Yang Z, Klionsky DJ . Eaten alive: a history of macroautophagy. Nat Cell Biol 2010; 12: 814–22.
Tanida I, Ueno T, Kominami E . Human light chain 3/MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. J Biol Chem 2004; 279: 47704–10.
Kroemer G, Marino G, Levine B . Autophagy and the integrated stress response. Mol Cell 2010; 40: 280–93.
Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res 2012; 72: 1321–31.
Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun 1997; 231: 509–13.
Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A . DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol 2004; 2: e362.
Lev N, Ickowicz D, Barhum Y, Lev S, Melamed E, Offen D . DJ-1 protects against dopamine toxicity. J Neural Transm 2009; 116: 151–60.
Cookson MR . Pathways to Parkinsonism. Neuron 2003; 37: 7–10.
Liu H, Wang M, Li M, Wang D, Rao Q, Wang Y, et al. Expression and role of DJ-1 in leukemia. Biochem Biophys Res Commun 2008; 375: 477–83.
MacKeigan JP, Clements CM, Lich JD, Pope RM, Hod Y, Ting JP . Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Cancer Res 2003; 63: 6928–34.
Ismail IA, Kang HS, Lee HJ, Kwon BM, Hong SH . 2′-Benzoyloxycinnamaldehyde-mediated DJ-1 upregulation protects MCF-7 cells from mitochondrial damage. Biol Pharm Bull 2012; 35: 895–902.
Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM . Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 2009; 87: 123–9.
McCoy MK, Cookson MR . DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy 2011; 7: 531–2.
Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H . DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 2004; 5: 213–8.
Opipari AW Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR . Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res 2004; 64: 696–703.
O'Donovan TR, O'Sullivan GC, McKenna SL . Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy 2011; 7: 509–24.
Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol 2012; 83: 16–26.
Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett 2011; 307: 141–8.
Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A . Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ 2007; 14: 500–10.
Liu YQ, Hu XY, Lu T, Cheng YN, Young CY, Yuan HQ, et al. Retigeric acid B exhibits antitumor activity through suppression of nuclear factor-kappaB signaling in prostate cancer cells in vitro and in vivo. PLoS One 2012; 7: e38000.
Liu H, Liu YQ, Liu YQ, Xu AH, Young CY, Yuan HQ, et al. A novel anticancer agent, retigeric acid B, displays proliferation inhibition, S phase arrest and apoptosis activation in human prostate cancer cells. Chem Biol Interact 2010; 188: 598–606.
Yuan H, Young CY, Tian Y, Liu Z, Zhang M, Lou H . Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol Cell Biochem 2010; 339: 253–62.
Zoratti M, Szabo I . The mitochondrial permeability transition. Biochim Biophys Acta 1995; 1241: 139–76.
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 2005; 65: 3336–46.
VanderWeele DJ, Zhou R, Rudin CM . Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol Cancer Ther 2004; 3: 1605–13.
Singh K, Matsuyama S, Drazba JA, Almasan A . Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress. Autophagy 2012; 8: 236–51.
Ferrin G, Linares CI, Muntane J . Mitochondrial drug targets in cell death and cancer. Curr Pharm Des 2011; 17: 2002–16.
Li ZY, Yang Y, Ming M, Liu B . Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun 2011; 414: 5–8.
Lemasters JJ, Qian T, Elmore SP, Trost LC, Nishimura Y, Herman B, et al. Confocal microscopy of the mitochondrial permeability transition in necrotic cell killing, apoptosis and autophagy. Biofactors 1998; 8: 283–5.
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1998; 1366: 177–96.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81273533 and 30925038), and the Natural Science Foundation of Shandong Province, China (2009ZRB01346).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Yq., Ji, Y., Li, Xz. et al. Retigeric acid B-induced mitophagy by oxidative stress attenuates cell death against prostate cancer cells in vitro. Acta Pharmacol Sin 34, 1183–1191 (2013). https://doi.org/10.1038/aps.2013.68
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.68
Keywords
This article is cited by
-
The pharmacological regulation of cellular mitophagy
Nature Chemical Biology (2017)
-
Jungermannenone A and B induce ROS- and cell cycle-dependent apoptosis in prostate cancer cells in vitro
Acta Pharmacologica Sinica (2016)
-
Malformin A1 promotes cell death through induction of apoptosis, necrosis and autophagy in prostate cancer cells
Cancer Chemotherapy and Pharmacology (2016)