Abstract
Aim:
To investigate the pharmacokinetics and disposition of simmitecan (L-P) that was a water-soluble ester prodrug of chimmitecan (L-2-Z) with potent anti-tumor activities in different experimental animals, and to assess its drug-drug interaction potential.
Methods:
SD rats were injected with a single iv bolus doses of L-P (3.75, 7.5 and 15 mg/kg). The pharmacokinetics, tissue distribution, excretion and metabolism of L-P and its active metabolite L-2-Z were studied through quantitative measurements and metabolite profiling with LC/MS. The binding of L-P and L-2-Z to rat plasma proteins was examined using an ultrafiltration method. Systemic exposures of beagle dogs to L-P as well as drug distribution in tumors of the nude mice xenograft model of human hepatic cancer SMMC-7721 cells were also examined. The metabolism of L-P by liver mcirosomal carboxylesterase in vitro was investigated in different species. The effects of L-P and L-2-Z on cytochrome P450 enzymes were examined using commercial screening kits.
Results:
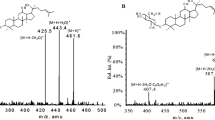
The in vivo biotransformation of L-P to L-2-Z showed a significant species difference, with a mean elimination half-life t1/2 of approximately 1.4 h in rats and 1.9 h in dogs. The systemic exposure levels of L-P and L-2-Z were increased in a dose-dependent manner. In rats, approximately 66% of L-P and 79% of L-2-Z were bound to plasma proteins. In rats and the nude mice bearing human hepatic cancers, most organ tissues had significantly higher concentrations of L-P than the corresponding plasma levels. In the tumor tissues, the L-P levels were comparable to those of plasma, whereas the L-2-Z levels were lower than the L-P levels. In rats, L-P was eliminated mainly via biliary excretion, but metabolism played an important role in elimination of the intact L-P. Finally, L-P and L-2-Z exerted moderate inhibition on the activity of CYP3A4 in vitro.
Conclusion:
L-P and L-2-Z have relatively short elimination half-lives and L-P is mainly eliminated via biliary excretion. The species difference in the conversion of L-P to L-2-Z and potential drug-drug interactions due to inhibition of CYP3A4 should be considered in further studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rivory L, Haaz M, Canal P, Lokiec F, Armand J, Robert J . Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin Cancer Res 1997; 3: 1261–6.
Haas N, LaCreta F, Walczak J, Hudes G, Brennan J, Ozols R, et al. Phase I/pharmacokinetic study of topotecan by 24-h continuous infusion weekly. Cancer Res 1994; 54: 1220–6.
Huang M, Gao H, Chen Y, Zhu H, Cai Y, Zhang X, et al. Chimmitecan, a novel 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin Cancer Res 2007; 13: 1298–307.
Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000; 17: 1278–83.
Guo B, Li C, Wang GJ, Chen LS . Rapid and direct measurement of free concentrations of highly protein-bound fluoxetine and its metabolite norfluoxetine in plasma. Rapid Commun Mass Spectrom 2006; 20: 39–47.
Lee KJ, Mower R, Hollenbeck T, Castelo J, Johnson N, Gordon P, et al. Modulation of nonspecific binding in ultrafiltration protein binding studies. Pharm Res 2003; 20: 1015–21.
Hu Z, Sun Y, Du F, Niu W, Xu F, Huang Y, et al. Accurate determination of the anticancer prodrug simmitecan and its active metabolite chimmitecan in various plasma samples based on immediate deactivation of blood carboxylesterases. J Chromatogr A 2011; 1218: 6646–53.
Kostiainen R, Kotiaho T, Kuuranne T, Auriola S . Liquid chromatography/atmospheric pressure ionization-mass spectrometry in drug metabolism studies. J Mass Spectrom 2003; 38: 357–72.
Davies B, Morris T . Physiological parameters in laboratory animals and humans. Pharm Res 1993; 10: 1093–5.
Combes O, Barre'J, Duche'J, Vernillet L, Archimbaud Y, Marietta M, et al. In vitro binding and partitioning of irinotecan (CPT-11) and its metabolite, SN-38, in human blood. Investig New Drugs 2000; 18: 1–5.
Rautio J, Kumpulainen H, Heimbach T, Oliyal R, Oh D, Jarvinen Y, et al. Prodrugs: design and clinical applications. Nat Rev 2008; 7: 255–70.
Stella V, Nti-addae K . Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev 2007; 59: 677–94.
Kaneda N, Nagata H, Furuta T, Yokokura T . Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res 1990; 50: 1715–20.
Hertzberg R, Caranfa M, Holden K, Jakas D, Gallagher G, Mattern M, et al. Modification of the hydroxy lactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. J Med Chem 1989; 32: 715–20.
Berry L, Wollenberg L, Zhao Z . Esterase activities in the blood, liver and intestine of several preclinical species and humans. Drug Metab Lett 2009; 3: 70–7.
Slatter J, Su P, Sams J, Schaaf L, Wienkers L . Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos 1997; 25: 1157–64.
Humerickhouse R, Lohrbach K, Li L, Bosron W, Dolan M . Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res 2000; 60: 1189–92.
Hamberg P, Donders R, ten Bokkel Huinink D . Central nervous system toxicity induced by irinotecan. J Natl Cancer Inst 2006; 98: 219.
Kovacic P . Mechanism of central nervous system toxicity by irinotecan. Med Hypotheses 2006; 67: 997.
Ehrich M, Veronesi B . Esterase comparison in neuroblastoma cells of human and rodent origin. Clin Exp Pharmacol Physiol 1995; 22: 328–86.
Zamboni WC, Houghton PJ, Thompson J . Altered irinotecan and SN-38 disposition after intravenous and oral administration of irinotecan in mice bearing human neuroblastoma xenografts. Clin Cancer Res 1998; 4: 455–62.
Takeda Y, Kobayashi K, Akiyama Y, Soma T, Handa S, Kudoh S, et al. Prevention of irinotecan (CPT-11)-induced diarrhea by oral alkalization combined with control of defecation in cancer patients. Int J Cancer 2001; 92: 269–75.
Marier J, Vachon P, Gritsas A, Zhang J, Moreau J, Ducharme M . Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther 2002; 302: 369–73.
Acknowledgements
This work was supported by the National Science & Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (Grant no 2009ZX09304-002, 2009ZX09301-001 and 2009ZX09102-020), the National Natural Science Foundation of China (Grant no 30873120) and the Chinese National Natural Science Foundation for Distinguished Young Scholars (Grant no 30925044). The authors would like to thank Dr Jian DING and Dr Ze-hong MIAO from the Shanghai Institute of Materia Medica for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Zy., Li, Xx., Du, Ff. et al. Pharmacokinetic evaluation of the anticancer prodrug simmitecan in different experimental animals. Acta Pharmacol Sin 34, 1437–1448 (2013). https://doi.org/10.1038/aps.2013.74
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.74