Abstract
Aim:
To develop a population pharmacokinetic model for the immunosuppressant ciclosporin in Chinese children with aplastic anemia and to identify covariates influencing ciclosporin pharmacokinetics.
Methods:
A total of 102 children with either acquired or congenital aplastic anemia aged 8.8±3.6 years (range 0.9–17.6 years) were included. Therapeutic drug monitoring (TDM) data for ciclosporin were collected. The population pharmacokinetic model of ciclosporin was described using the nonlinear mixed-effects modeling (NONMEM) VI software. The final model was validated using bootstrap and normalized prediction distribution errors.
Results:
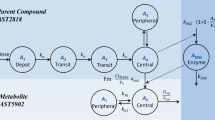
A one-compartment model with first-order absorption and elimination was developed. The estimated CL/F was 15.1, which was lower than those of children receiving stem cell or kidney transplant reported in the West (16.9–29.3). The weight normalized CL/F was 0.45 (range: 0.27–0.70) Lh−1·kg−1. The covariate analysis identified body weight, serum creatinine and concomitant administration of the anabolic steroid stanozolol as individual factors influencing the CL/F of ciclosporin.
Conclusion:
Our model could be used to optimize the ciclosporin dosing regimen in Chinese children with aplastic anemia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Li FP, Alter BP, Nathan DG . The mortality of acquired aplastic anemia in children. Blood 1972; 40: 153–62.
Maschan A, Bogatcheva N, Kryjanovskii O, Shneider M, Litvinov D, Mitiushkina T, et al. Results at a single centre of immunosuppression with cyclosporine A in 66 children with aplastic anaemia. Br J Haematol 1999; 106: 967–70.
Yee GC . Recent advances in cyclosporine pharmacokinetics. Pharmacotherapy 1991; 11: 130S–134S.
del Mar Fernández De Gatta M, Santos-Buelga D, Domínguez-Gil A, García MJ . Immunosuppressive therapy for paediatric transplant patients: pharmacokinetic considerations. Clin Pharmacokinet 2002; 41: 115–35.
Zhao W, Fakhoury M, Jacqz-Aigrain E . Developmental pharmacogenetics of immunosuppressants in pediatric organ transplantation. Ther Drug Monit 2010; 32: 688–99.
Zheng QS, Li LJ . Pharmacometrics: a quantitative tool of pharmacological research. Acta Pharmacol Sin 2012; 33: 1337–8.
Fanta S, Jönsson S, Backman JT, Karlsson MO, Hoppu K . Developmental pharmacokinetics of ciclosporin — a population pharmacokinetic study in paediatric renal transplant candidates. Br J Clin Pharmacol 2007; 64: 772–84.
Irtan S, Saint-Marcoux F, Rousseau A, Zhang D, Leroy V, Marquet P, et al. Population pharmacokinetics and bayesian estimator of cyclosporine in pediatric renal transplant patients. Ther Drug Monit 2007; 29: 96–102.
Willemze AJ, Cremers SC, Schoemaker RC, Lankester AC, den Hartigh J, Burggraaf J, et al. Ciclosporin kinetics in children after stem cell transplantation. Br J Clin Pharmacol 2008; 66: 539–45.
Bing H, Siyi Y, Wei Z, Jian L, Minghui D, Li J, et al. The use of anti-human T lymphocyte porcine immunoglobulin and cyclosporine a to treat patients with acquired severe aplastic anemia. Acta Haematol 2010; 124: 245–50.
Anderson BJ, Holford NH . Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32.
Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther 2009; 86: 609–18.
Lindbom L, Ribbing J, Jonsson EN . Perl-speaks-NONMEM (PsN) — a Perl module for NONMEM related programming. Comput Methods Programs Biomed 2004; 75: 85–94.
Brendel K, Comets E, Laffont C, Laveille C, Mentré F . Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 2006; 23: 2036–49.
Comets E, Brendel K, Mentré F . Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 2008; 90: 154–66.
Zhao W, Piana C, Danhof M, Burger D, Pasqua OD, Jacqz-Aigrain E . Population pharmacokinetics of abacavir in infants, toddlers and children. Br J Clin Pharmacol 2012. DOI: 10.1111/bcp.12024.
Blasco H, Senecal D, Le Gouge A, Pinard E, Benz-de Bretagne I, Colombat P, et al. Influence of methotrexate exposure on outcome in patients treated with MBVP chemotherapy for primary central nervous system lymphoma. Br J Clin Pharmacol 2010; 70: 367–75.
Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL . Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral) 1 in organ transplantation. Drugs 2001; 61: 1957–2016.
Saint-Marcoux F, Marquet P, Jacqz-Aigrain E, Bernard N, Thiry P, Le Meur Y, et al. Patient characteristics influencing ciclosporin pharmacokinetics and accurate Bayesian estimation of ciclosporin exposure in heart, lung and kidney transplant patients. Clin Pharmacokinet 2006; 45: 905–22.
Nolin TD, Naud J, Leblond FA, Pichette V . Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 2008; 83: 898–903.
Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J . Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol 2006; 17: 2363–7.
Benfield MR, Stablein D, Tejani A . Trends in immunosuppressive therapy: a report of the north American pediatric renal transplant cooperative study (NAPRTCS). Pediatr Transplant 1999; 3: 27–32.
Ettenger RB . New immunosuppressive agents in pediatric renal transplantation. Transplant Proc 1998; 30: 1956–8.
Nakamura H, Nakasa H, Ishii I, Ariyoshi N, Igarashi T, Ohmori S, et al. Effects of endogenous steroids on CYP3A4-mediated drug metabolism by human liver microsomes. Drug Metab Dispos 2002; 30: 534–40.
Lam S, Partovi N, Ting LS, Ensom MH . Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother 2008; 42: 1037–47.
Zhou H, Gao Y, Cheng XL, Li ZD . Population pharmacokinetics of cyclosporine A based on NONMEM in Chinese allogeneic hematopoietic stem cell transplantation recipients. Eur J Drug Metab Pharmacokinet 2012; 37: 271–8.
Acknowledgements
We thank Dingtai Ren for his great support in recording information and sample treatments and David Shen for his kindly help in our population pharmacokinetic experiments. We also sincerely thank all the participating patients and their families for their cooperation.
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (Y2080183), the National Natural Science Foundation of China (81273607), the Science and Technologies Foundation of China (2009ZX09304) and the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2013ZX09303003). Doctor Wei Zhao and Professor Evelyne Jacqz-Aigrain received support from the European commission for their pediatric pharmacology collaborative work in China (FP7 Global research in pediatrics GRIP, grant agreement No 261060).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ni, Sq., Zhao, W., Wang, J. et al. Population pharmacokinetics of ciclosporin in Chinese children with aplastic anemia: effects of weight, renal function and stanozolol administration. Acta Pharmacol Sin 34, 969–975 (2013). https://doi.org/10.1038/aps.2013.9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2013.9
Keywords
This article is cited by
-
Population pharmacokinetics of cyclosporine in Chinese children receiving hematopoietic stem cell transplantation
Acta Pharmacologica Sinica (2019)