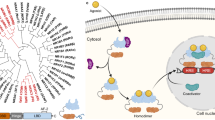
Abstract
Nuclear receptors (NRs) are members of a large superfamily of evolutionarily related transcription factors that control a plethora of biological processes. NRs orchestrate complex events such as development, organ homeostasis, metabolism, immune function, and reproduction. Approximately one-half of the 48 human NRs have been shown to act as ligand-regulated transcription factors and respond directly to a large variety of endogenous hormones and metabolites that are generally hydrophobic and small in size (eg, retinoic acid or estradiol). The second half of the NR family comprises the so-called orphan receptors, for which regulatory ligands are still unknown or may not exist despite the presence of a C-terminal ligand-binding domain, which is the hallmark of all NRs. Several chemicals released into the environment (eg, bisphenols, phthalates, parabens, etc) share some physicochemical properties with natural ligands, allowing them to bind to NRs and activate or inhibit their action. Collectively referred to as endocrine disruptors or endocrine-disrupting chemicals (EDCs), these environmental pollutants are highly suspected to cause a wide range of developmental, reproductive, neurological, or metabolic defects in humans and wildlife. Crystallographic studies are revealing unanticipated mechanisms by which chemically diverse EDCs interact with the ligand-binding domain of NRs. These studies thereby provide a rational basis for designing novel chemicals with lower impacts on human and animal health. In this review, we provide a structural and mechanistic view of endocrine disrupting action using estrogen receptors α and β, (ERα/β), peroxisome proliferator activated receptor γ (PPARγ), and their respective environmental ligands as representative examples.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Germain P, Staels B, Dacquet C, Spedding M, Laudet V . Overview of nomenclature of nuclear receptors. Pharmacol Rev 2006; 58: 685–704.
Gronemeyer H, Gustafsson JA, Laudet V . Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 2004; 3: 950–64.
Rastinejad F, Huang P, Chandra V, Khorasanizadeh S . Understanding nuclear receptor form and function using structural biology. J Mol Endocrinol 2013; 51: T1–T21.
Li Y, Lambert MH, Xu HE . Activation of nuclear receptors: a perspective from structural genomics. Structure 2003; 11: 741–6.
Bourguet W, Germain P, Gronemeyer H . Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci 2000; 21: 381–8.
Hughes TS, Chalmers MJ, Novick S, Kuruvilla DS, Chang MR, Kamenecka TM, et al. Ligand and receptor dynamics contribute to the mechanism of graded PPARgamma agonism. Structure 2011; 20: 139–50.
Nettles KW, Greene GL . Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 2005; 67: 309–33.
Nahoum V, Perez E, Germain P, Rodriguez-Barrios F, Manzo F, Kammerer S, et al. Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc Natl Acad Sci U S A 2007; 104: 17323–8.
Bourguet W, de Lera AR, Gronemeyer H . Inverse agonists and antagonists of retinoid receptors. Methods Enzymol 2010; 485: 161–95.
Germain P, Gaudon C, Pogenberg V, Sanglier S, Van Dorsselaer A, Royer CA, et al. Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists. Chem Biol 2009; 16: 479–89.
le Maire A, Teyssier C, Erb C, Grimaldi M, Alvarez S, de Lera AR, et al. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat Struct Mol Biol 2010; 17: 801–7.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009; 30: 293–342.
Schug TT, Janesick A, Blumberg B, Heindel JJ . Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 2011; 127: 204–15.
De Coster S, van Larebeke N . Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health 2012; 2012: 713696.
le Maire A, Bourguet W, Balaguer P . A structural view of nuclear hormone receptor: endocrine disruptor interactions. Cell Mol Life Sci 2010; 67: 1219–37.
Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 2006; 58: 773–81.
Jensen EV, Jordan VC . The estrogen receptor: a model for molecular medicine. Clin Cancer Res 2003; 9: 1980–9.
Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ . Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006; 126: 789–99.
Couse JF, Korach KS . Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 1999; 20: 358–417.
Levin ER, Pietras RJ . Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat 2008; 108: 351–61.
Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A 2000; 97: 5936–41.
Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol 2003; 17: 203–8.
Ellem SJ, Risbridger GP . The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci 2009; 1155: 174–86.
Delfosse V, Grimaldi M, Cavailles V, Balaguer P, Bourguet W . Structural and functional profiling of estrogen receptors environmental ligands. Environ Health Perspect 2014; 122: 1306–13.
Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997; 138: 863–70.
Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J 1999; 18: 4608–18.
Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife (Cambridge) 2014; 3: e02057.
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM . Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 2009; 30: 75–95.
Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, et al. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A 2012; 109: 14930–5.
Ikeda K, Arao Y, Otsuka H, Nomoto S, Horiguchi H, Kato S, et al. Terpenoids found in the umbelliferae family act as agonists/antagonists for ER(alpha) and ERbeta: differential transcription activity between ferutinine-liganded ER(alpha) and ERbeta. Biochem Biophys Res Commun 2002; 291: 354–60.
Nettles KW, Sun J, Radek JT, Sheng S, Rodriguez AL, Katzenellenbogen JA, et al. Allosteric control of ligand selectivity between estrogen receptors alpha and beta: implications for other nuclear receptors. Mol Cell 2004; 13: 317–27.
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252–63.
Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R . Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci 2006; 79: 1160–9.
Multigner L, Ndong JR, Giusti A, Romana M, Delacroix-Maillard H, Cordier S, et al. Chlordecone exposure and risk of prostate cancer. J Clin Oncol 2010; 28: 3457–62.
Boucher O, Simard MN, Muckle G, Rouget F, Kadhel P, Bataille H, et al. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology 2013; 35: 162–8.
Dallaire R, Muckle G, Rouget F, Kadhel P, Bataille H, Guldner L, et al. Cognitive, visual, and motor development of 7-month-old Guadeloupean infants exposed to chlordecone. Environ Res 2012; 118: 79–85.
Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 2006; 58: 726–41.
Varga T, Czimmerer Z, Nagy L . PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011; 1812: 1007–22.
Desvergne B, Wahli W . Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20: 649–88.
Berger J, Moller DE . The mechanisms of action of PPARs. Annu Rev Med 2002; 53: 409–35.
Tontonoz P, Spiegelman BM . Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008; 77: 289–312.
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19: 557–66.
Grygiel-Gorniak B . Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications — a review. Nutr J 2014; 13: 17.
Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, et al. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol 2008; 15: 865–7.
Lamers C, Schubert-Zsilavecz M, Merk D . Therapeutic modulators of peroxisome proliferator-activated receptors (PPAR): a patent review (2008-present). Expert Opin Ther Pat 2012; 22: 803–41.
Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 2010; 466: 451–6.
Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, et al. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature 2011; 477: 477–81.
Hughes TS, Giri PK, de Vera IM, Marciano DP, Kuruvilla DS, Shin Y, et al. An alternate binding site for PPARgamma ligands. Nat Commun 2013; 5: 3571.
Swedenborg E, Ruegg J, Makela S, Pongratz I . Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol 2009; 43: 1–10.
le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, et al. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep 2009; 10: 367–73.
Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, et al. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ Health Perspect 2011; 119: 1227–32.
Janesick A, Blumberg B . Minireview: PPARgamma as the target of obesogens. J Steroid Biochem Mol Biol 2011; 127: 4–8.
Grun F, Blumberg B . Endocrine disrupters as obesogens. Mol Cell Endocrinol 2009; 304: 19–29.
Janesick A, Blumberg B . Obesogens, stem cells and the developmental programming of obesity. Int J Androl 2012; 35: 437–48.
Grun F, Blumberg B . Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006; 147 (6 Suppl): S50–5.
Issemann I, Green S . Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990; 347: 645–50.
Zoete V, Grosdidier A, Michielin O . Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta 2007; 1771: 915–25.
Waku T, Shiraki T, Oyama T, Maebara K, Nakamori R, Morikawa K . The nuclear receptor PPARgamma individually responds to serotonin- and fatty acid-metabolites. Embo J 2010; 29: 3395–407.
Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol 2008; 15: 924–31.
Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 2006; 20: 2141–55.
Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J . Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 2005; 67: 766–74.
Fent K . Ecotoxicology of organotin compounds. Crit Rev Toxicol 1996; 26: 1–117.
Appel KE . Organotin compounds: toxicokinetic aspects. Drug Metab Rev 2004; 36 (3-4): 763–86.
Antizar-Ladislao B . Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ Int 2008; 34: 292–308.
Fent K . Ecotoxicological problems associated with contaminated sites. Toxicol Lett 2003; 140-141: 353–65.
Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S . Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol 2010; 58: 901–7.
Gumy C, Chandsawangbhuwana C, Dzyakanchuk AA, Kratschmar DV, Baker ME, Odermatt A . Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PLoS ONE 2008; 3: e3545.
de Wit CA, Herzke D, Vorkamp K . Brominated flame retardants in the Arctic environment — trends and new candidates. Sci Total Environ 2009; 408: 2885–918.
Darnerud PO . Toxic effects of brominated flame retardants in man and in wildlife. Environ Int 2003; 29: 841–53.
Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, et al. Exposure assessment of French women and their newborns to tetrabromobisphenol–A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008; 73: 1036–41.
Shi H, Qian L, Guo S, Zhang X, Liu J, Cao Q . Teratogenic effects of tetrabromobisphenol A on Xenopus tropicalis embryos. Comp Biochem Physiol C Toxicol Pharmacol 2009; 152: 62–8.
Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, et al. Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci 2011; 122: 372–82.
Calleri E, Pochetti G, Dossou KS, Laghezza A, Montanari R, Capelli D, et al. Resveratrol and its metabolites bind to PPARs. Chembiochem 2014; 15: 1154–60.
Zhang H, Xu X, Chen L, Chen J, Hu L, Jiang H, et al. Molecular determinants of magnolol targeting both RXRalpha and PPARgamma. PLoS One 2011; 6: e28253.
Puhl AC, Bernardes A, Silveira RL, Yuan J, Campos JL, Saidemberg DM, et al. Mode of peroxisome proliferator-activated receptor gamma activation by luteolin. Mol Pharmacol 2012; 81: 788–99.
Fremont L . Biological effects of resveratrol. Life Sci 2000; 66: 663–73.
Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C . Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther 2001; 296: 181–7.
Havsteen BH . The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002; 96: 67–202.
Choi SS, Cha BY, Lee YS, Yonezawa T, Teruya T, Nagai K, et al. Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci 2009; 84: 908–14.
Chen J, Wang J, Shao J, Gao Y, Xu J, Yu S, et al. The unique pharmacological characteristics of mifepristone (RU486): from terminating pregnancy to preventing cancer metastasis. Med Res Rev 2014 Sep; 34: 979–1000.
Scott PD, Bartkow M, Blockwell SJ, Coleman HM, Khan SJ, Lim R, et al. An assessment of endocrine activity in Australian rivers using chemical and in vitro analyses. Environ Sci Pollut Res Int 2014; 21: 12951–67.
Lin S, Han Y, Shi Y, Rong H, Zheng S, Jin S, et al. Revealing a steroid receptor ligand as a unique PPARgamma agonist. Cell Res 2012; 22: 746–56.
Kauppi B, Jakob C, Farnegardh M, Yang J, Ahola H, Alarcon M, et al. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J Biol Chem 2003; 278: 22748–54.
Raaijmakers HC, Versteegh JE, Uitdehaag JC . The X-ray structure of RU486 bound to the progesterone receptor in a destabilized agonistic conformation. J Biol Chem 2009; 284: 19572–9.
Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997; 389: 753–8.
Manas ES, Xu ZB, Unwalla RJ, Somers WS . Understanding the selectivity of genistein for human estrogen receptor-beta using X-ray crystallography and computational methods. Structure 2004; 12: 2197–207.
Nettles KW, Bruning JB, Gil G, Nowak J, Sharma SK, Hahm JB, et al. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nat Chem Biol 2008; 4: 241–7.
Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998; 95: 927–37.
Mocklinghoff S, Rose R, Carraz M, Visser A, Ottmann C, Brunsveld L . Synthesis and crystal structure of a phosphorylated estrogen receptor ligand binding domain. Chembiochem 2010; 11: 2251–4.
Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 1998; 395: 137–43.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delfosse, V., Maire, A., Balaguer, P. et al. A structural perspective on nuclear receptors as targets of environmental compounds. Acta Pharmacol Sin 36, 88–101 (2015). https://doi.org/10.1038/aps.2014.133
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.133
Keywords
This article is cited by
-
Natural compounds targeting nuclear receptors for effective cancer therapy
Cancer and Metastasis Reviews (2023)
-
Risk of thyroid cancer and benign nodules associated with exposure to parabens among Chinese adults in Wuhan, China
Environmental Science and Pollution Research (2022)
-
Insights into the activation mechanism of human estrogen-related receptor γ by environmental endocrine disruptors
Cellular and Molecular Life Sciences (2019)
-
Confirmation of high-throughput screening data and novel mechanistic insights into VDR-xenobiotic interactions by orthogonal assays
Scientific Reports (2018)
-
Identification of a novel selective PPARγ ligand with a unique binding mode and improved therapeutic profile in vitro
Scientific Reports (2017)