Abstract
Aim:
Glycogen synthase kinase 3β (GSK-3β) plays a crucial role in hepatic biology, including liver development, regeneration, proliferation and carcinogenesis. In this study we investigated the role of GSK-3β in regulation of growth of hepatic oval cells in vitro and in liver regeneration in partially hepatectomized rats.
Methods:
WB-F344 cells, the rat hepatic stem-like epithelial cells, were used as representative of oval cells. Cell viability was examined using a WST-8 assay. The cells were transfected with a recombinant lentivirus expressing siRNA against GSK-3β (GSK-3βRNAiLV) or a lentivirus that overexpressed GSK-3β (GC-GSK-3βLV). Adult rats underwent partial (70%) hepatectomy, and liver weight and femur length were measured at d 7 after the surgery. The expression of GSK-3β, phospho-Ser9-GSK-3β, β-catenin and cyclin D1 was examined with immunoblotting assays or immunohistochemistry.
Results:
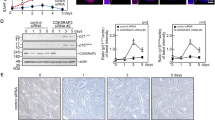
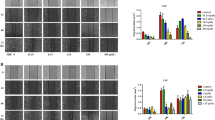
Treatment of WB-F344 cells with the GSK-3β inhibitor SB216763 (5 and 10 μmol/L) dose-dependently increased the levels of phospho-Ser9-GSK-3β, but not the levels of total GSK-3β, and promoted the cell proliferation. Knockout of GSK-3β with GSK-3βRNAiLV increased the cell proliferation, whereas overexpression of GSK-3β with GC-GSK-3βLV decreased the proliferation. Both SB216763 and GSK-3βRNAiLV significantly increased the levels of β-catenin and cyclin D1 in the cells, whereas GSK-3β overexpression decreased their levels. In rats with a partial hepatectomy, administration of SB216763 (2 mg/kg, ip) significantly increased the number of oval cells, the levels of phospho-Ser9-GSK-3β, β-catenin and cyclin D1 in liver, as well as the ratio of liver weight to femur length at d 7 after the surgery.
Conclusion:
GSK-3β suppresses the proliferation of hepatic oval cells by modulating the Wnt/β-catenin signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fausto N, Campbell JS . The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 2003; 120: 117–30.
Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK . Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology 1998; 27: 433–45.
Paku S, Nagy P, Kopper L, Thorgeirsson SS . 2-Acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology 2004; 39: 1353–61.
Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M . The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci U S A 2003; 100: 11881–8.
Lemire JM, Shiojiri N, Fausto N . Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol 1991; 139: 535–52.
Dabeva MD, Shafritz DA . Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol 1993; 143: 1606–20.
Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS . Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis 1996; 17: 2143–51.
Roskams TA, Libbrecht L, Desmet VJ . Progenitor cells in diseased human liver. Semin Liver Dis 2003; 23: 385–96.
Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA . Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol Gastrointest Liver Physiol 2006; 291: G45–54.
Woodgett JR, Cohen P . Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta 1984; 788: 339–47.
Woodgett JR . Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 1990; 9: 2431–8.
Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A . Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol 2009; 156: 885–98.
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR . Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000; 406: 86–90.
Jin J, Wang GL, Shi X, Darlington GJ, Timchenko NA . The age-associated decline of glycogen synthase kinase 3beta plays a critical role in the inhibition of liver regeneration. Mol Cell Biol 2009; 29: 3867–80.
Chen H, Yang S, Yang Z, Ma L, Jiang D, Mao J, et al. Inhibition of GSK-3beta decreases NF-kappaB-dependent gene expression and impairs the rat liver regeneration. J Cell Biochem 2007; 102: 1281–9.
Desbois-Mouthon C, Blivet-Van EMJ, Beurel E, Boissan M, Delelo R, Cadoret A, et al. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology 2002; 36: 1528–36.
Sekiya S, Suzuki A . Glycogen synthase kinase 3 beta-dependent Snail degradation directs hepatocyte proliferation in normal liver regeneration. Proc Natl Acad Sci U S A 2011; 108: 11175–80.
Ito H, Kamiya A, Ito K, Yanagida A, Okada K, Nakauchi H . In vitro expansion and functional recovery of mature hepatocytes from mouse adult liver. Liver Int 2012; 32: 592–601.
Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, et al. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol 1997; 151: 353–9.
Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y . In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation 2002; 69: 209–15.
Thorgeirsson SS, Grisham JW . Overview of recent experimental studies on liver stem cells. Semin Liver Dis 2003; 23: 303–12.
Tsao MS, Smith JD, Nelson KG, Grisham JW . A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res 1984; 154: 38–52.
Bisgaard HC, Nagy P, Santoni-Rugiu E, Thorgeirsson SS . Proliferation, apoptosis, and induction of hepatic transcription factors are characteristics of the early response of biliary epithelial (oval) cells to chemical carcinogens. Hepatology 1996; 23: 62–70.
Alison MR, Golding M, Sarraf CE, Edwards RJ, Lalani EN . Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology 1996; 110: 1182–90.
Duncan AW, Dorrell C, Grompe M . Stem cells and liver regeneration. Gastroenterology 2009; 137: 466–81.
Meijer L, Flajolet M, Greengard P . Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci 2004; 25: 471–80.
Jope RS, Johnson GV . The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 2004; 29: 95–102.
Plumpe J, Malek NP, Bock CT, Rakemann T, Manns MP, Trautwein C . NF-kappaB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol 2000; 278: G173–83.
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P . Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 1996; 272: 1023–6.
Chen RH, Ding WV, McCormick F . Wnt signaling to betacatenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem 2000; 275: 7894–9.
Patel S, Doble B, Woodgett JR . Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem Soc Trans 2004; 32: 803–8.
Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, et al. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol 2008; 73: 59–66.
Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, et al. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 2007; 133: 1579–91.
Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP . Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology 2008; 47: 288–95.
Zhang Y, Li XM, Zhang FK, Wang BE . Activation of canonical Wnt signaling pathway promotes proliferation and self-renewal of rat hepatic oval cell line WB-F344 in vitro. World J Gastroenterol 2008; 14: 6673–80.
Itoh T, Kamiya Y, Okabe M, Tanaka M, Miyajima A . Inducible expression of Wnt genes during adult hepatic stem/progenitor cell response. FEBS Lett 2009; 583: 777–81.
Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT . The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 1996; 10: 1443–54.
van Es JH, Barker N, Clevers H . You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 2003; 13: 28–33.
Moon RT, Bowerman B, Boutros M, Perrimon N . The promise and perils of Wnt signaling through beta-catenin. Science 2002; 296:1644–6.
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH . Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10: 55–63.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116: 1175–86.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No 30700800) and Zhejiang Extremely Key Subject of Surgery. We thank Ka-te HUANG and Li WAN for their technical assistance with the immunohistochemistry.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ji, Xk., Xie, Yk., Zhong, Jq. et al. GSK-3β suppresses the proliferation of rat hepatic oval cells through modulating Wnt/β-catenin signaling pathway. Acta Pharmacol Sin 36, 334–342 (2015). https://doi.org/10.1038/aps.2014.150
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.150
Keywords
This article is cited by
-
Wnt–β-catenin signalling in liver development, health and disease
Nature Reviews Gastroenterology & Hepatology (2019)
-
GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats
Molecular Neurobiology (2016)
-
Omega-3 fatty acids induce Ca2+ mobilization responses in human colon epithelial cell lines endogenously expressing FFA4
Acta Pharmacologica Sinica (2015)