Abstract
Aim:
Tramadol is an atypical opioid analgesic with low potential for tolerance and addiction. However, its opioid activity is much lower than classic opiates such as morphine. To develop novel analgesic and further explore the structure activity relationship (SAR) of tramadol skeleton.
Methods:
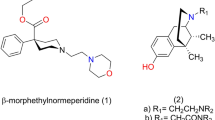
Based on a three-dimensional (3D) structure superimposition and molecular docking study, we found that M1 (the active metabolite of tramadol) and morphine have common pharmacophore features and similar binding modes at the μ opioid receptor in which the substituents on the nitrogen atom of both compounds faced a common hydrophobic pocket formed by Trp2936.48 and Tyr3267.43. In this study, N-phenethylnormorphine was docked to the μ opioid receptor. It was found that the N-substituted group of N-phenethylnormorphine extended into a hydrophobic pocket formed by Trp2936.48 and Tyr3267.43. This hydrophobic interaction may contribute to the improvement of its opioid activities as compared with morphine. The binding modes of M1, morphine and N-phenethylnormorphine overlapped, indicating that the substituent on the nitrogen atoms of the three compounds may adopt common orientations. A series of N-phenylalkyl derivatives from the tramadol scaffold were designed, synthesized and assayed in order to generate a new type of analgesics.
Results:
As a result, compound 5b was identified to be an active candidate from these compounds. Furthermore, the binding modes of 5b and morphine derivatives in the μ opioid receptor were comparatively studied.
Conclusion:
Unlike morphine-derived structures in which bulky N-substitution is associated with improved opioid-like activities, there seems to be a different story for tramadol, suggesting the potential difference of SAR between these compounds. A new type of interaction mechanism in tramadol analogue (5b) was discovered, which will help advance potent tramadol-based analgesic design.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grond S, Sablotzki A . Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43: 879–923.
Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50: 1842–6.
Harati Y, Gooch C, Swenson M, Edelman SV, Greene D, Raskin P, et al. Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. J Diabetes Complications 2000; 14: 65–70.
Barber J . Examining the use of tramadol hydrochloride as an antidepressant. Exp Clin Psychopharmacol 2011; 19: 123–30.
Gobel H, Stadler T . Treatment of post-herpes zoster pain with tramadol. Results of an open pilot study versus clomipramine with or without levomepromazine. Drugs 1997; 53: 34–9.
Boureau F, Legallicier P, Kabir-Ahmadi M . Tramadol in post-herpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Pain 2003; 104: 323–31.
Wu T, Yue X, Duan X, Luo DY, Cheng Y, Tian Y, et al. Efficacy and safety of tramadol for premature ejaculation: a systematic review and meta-analysis. Urology 2012; 80: 618–24.
Wong BLK, Malde S . The use of tramadol “on-demand” for premature ejaculation: a systematic review. Urology 2013; 81: 98–103.
Langley PC, Patkar AD, Boswell KA, Benson CJ, Schein JR . Adverse event profile of tramadol in recent clinical studies of chronic osteoarthritis pain. Curr Med Res Opin 2010; 26: 239–51.
Keating GM . Tramadol sustained-release capsules. Drugs 2006; 66: 223–30.
Gobbi M, Moia M, Pirona L, Ceglia I, Reyes-Parada M, Scorza C, et al. p-Methylthioamphetamine and 1-(m-chlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of action at 5-HT nerve endings in vitro. J Neurochem 2002; 82: 1435–43.
Reimann W, Schneider F . Induction of 5-hydroxytryptamine release by tramadol, fenfluramine and reserpine. Eur J Pharmacol 1998; 349: 199–203.
Gillen C, Haurand M, Kobelt DJ, Wnendt S . Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol 2000; 362: 116–21.
Yu Z, Ma YC, Ai J, Chen DQ, Zhao DM, Wang X, et al. Energetic factors determining the binding of type I inhibitors to c-Met kinase: experimental studies and quantum mechanical calculations. Acta Pharmacol Sin 2013; 34: 1475–83.
Jones G, Willett P, Glen RC, Leach AR, Taylor R . Development and validation of a genetic algorithm for flexible docking. J Mol Biol 1997; 267: 727–48.
Shao L, Abolin C, Hewitt MC, Koch P, Varney M . Derivatives of tramadol for increased duration of effect. Bioorg Med Chem Lett 2006; 16: 691–4.
Shao L, Wang F, Hewitt MC, Barberich TJ . mu-Opioid/5-HT4 dual pharmacologically active agents-efforts towards an effective opioid analgesic with less GI and respiratory side effects (Part I). Bioorg Med Chem Lett 2009; 19: 5679–83.
Buschmann H, Christoph T, Maul C, Sundermann B, editors. Analgesics: from chemistry and pharmacology to clinical application. Germany: Heppenheim; 2002.
Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, et al. Structure of the delta-opioid receptor bound to naltrindole. Nature 2012; 485: 400–4.
Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012; 485: 321–6.
Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012; 485: 327–32.
Acknowledgements
This work was supported by the Science and Technology Commission of Shanghai Municipality (No 14431990500) and the National Natural Science Foundation of China (No 81473136 and 30901857).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, Q., Qian, Yy., Xu, Xj. et al. Design, synthesis and biological evaluation of N-phenylalkyl-substituted tramadol derivatives as novel μ opioid receptor ligands. Acta Pharmacol Sin 36, 887–894 (2015). https://doi.org/10.1038/aps.2014.171
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.171
Keywords
This article is cited by
-
Modulation of social and depression behaviors in cholestatic and drug-dependent mice: possible role of opioid receptors
Journal of Diabetes & Metabolic Disorders (2022)