Abstract
Aim:
Biomarkers and image markers of Alzheimer's disease (AD), such as cerebrospinal fluid Aβ42 and p-tau, are effective predictors of cognitive decline or dementia. The aim of this study was to integrate these markers with a disease progression model and to identify their abnormal ranges.
Methods:
The data of 395 participants, including 86 normal subjects, 108 early mild cognitive impairment (EMCI) subjects, 120 late mild cognitive impairment (LMCI) subjects, and 81 AD subjects were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database. For the participants, baseline and long-term data on cerebrospinal fluid Aβ42 and p-tau, hippocampal volume, and ADAS-cog were available. Various linear and nonlinear models were tested to determine the associations among the ratio of Aβ42 to p-tau (the Ratio), hippocampal volume and ADAS-cog.
Results:
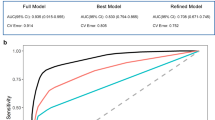
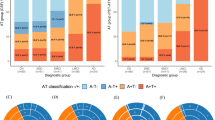
The most likely models for the Ratio, hippocampal volume, and ADAS-cog (logistic, Emax, and linear models, respectively) were used to construct the final model. Baseline disease state had an impact on all the 3 endpoints (the Ratio, hippocampal volume, and ADAS-cog), while APOEε4 genotype and age only influence the Ratio and hippocampal volume.
Conclusion:
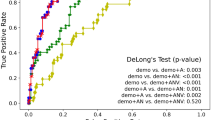
The Ratio can be used to identify the disease stage for an individual, and clinical measures integrated with the Ratio improve the accuracy of mild cognitive impairment (MCI) to AD conversion forecasting.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nelson PT, Braak H, Markesbery WR . Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol 2009; 68: 1–14.
Sperling RA, Aisen PS, Beckett LA, Bennet DAD, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer Dement 2011; 7: 280–92.
Morris JC, Storandt M, McKeel DWJ, Rubin EH, Price JL, Grant EA, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for pre-symptomatic and very mild Alzheimer's disease. Neurology 1996; 46: 707–19.
Holford NH, Peace KE . Methodologic aspects of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci U S A 1992; 89: 11466–70.
Samtani MN, Farnum M, Lobanov V, Yang E, Raghavan N, Dibernardo A, et al. An improved model for disease progression in patients from the Alzheimer's disease neuroimaging initiative. J Clin Pharmacol 2011; 52: 629–44.
Gomeni R, Simeoni M, Zvartau-Hind M, Irizarry MC, Austin D, Gold M . Modeling Alzheimer's disease progression using the disease system analysis approach. Alzheimers Dement 2012; 8: 39–50.
Mould DR . Models for disease progression: new approaches and uses. Clin Pharmacol Therapeut 2012; 92: 125–31.
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119–28.
Mouiha A, Duchesne S . Toward a dynamic biomarker model in Alzheimer's disease. J Alzheimers Dis 2012; 30: 91–100.
Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol 2003; 60: 1696–702.
Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ . Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov 2007; 6: 295–303.
Liu SJ, Wang JZ . Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol Sin 2002; 23: 183–7.
Hochfeld WE, Lee S, Rubinsztein DC . Therapeutic induction of autophagy to modulate neurodegenerative disease progression. Acta Pharmacol Sin 2013; 34: 600–4.
Glenner GG, Wong CW . Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 1984; 122: 1131–5.
Hardy J, Selkoe DJ . The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002; 297: 353–6.
Wang J, Wang Z . Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin 2006; 27: 41–9.
Duyckaerts C, Uchihara T, Seilhean D, He Y, Hauw JJ . Dissociation of Alzheimer type pathology in a disconnected piece of cortex. Acta Neuropathol 1997; 93: 501–7.
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006; 59: 512–9.
Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM . Cerebrospinal fluid tau/beta-amyloid42 ratio as a prediction of cognitive decline in non-demented older adults. Arch Neurol 2007; 64: 343–9.
Price JL, Morris JC . Tangles and plaques in non-demented aging and “preclinical” Alzheimer's disease. Ann Neurol 1999; 45: 358–68.
He L, Lu J, Yue Z . Autophagy in ageing and ageing-associated diseases. Acta Pharmacol Sin 2013; 34: 605–11.
Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR . A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000; 47: 36–45.
Han Y S, Zheng WH, Bastianetto S, Chabot JG, Quirion R . Neuroprotective effects of resveratrol against β-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol 2004; 141: 997–1005.
Rong XF, Wang XL . An overview of biomarkers in Alzheimer's disease. Yao Xue Xue Bao 2012; 47: 551–7.
Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 2006; 63: 693–9.
Kester MI, van der Flier WM, Mandic G, Blankenstein MA, Scheltens P, Muller M . Joint effect of hypertension and APOE genotype on CSF biomarkers for Alzheimer's disease. J Alzheimers Dis 2010; 20: 1083–90.
Caroli A, Frisoni GB . The Alzheimer's disease neuroimaging initiative. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's disease neuroimaging initiative cohort. Neurobiol Aging 2010; 31: 1263–74.
Palmqvist S, Hertze J, Minthon L, Wattmo C, Zetterberg H, Blennow K, et al. Comparison of brief cognitive tests and CSF biomarkers in predicting Alzheimer's disease in mild cognitive impairment: six-year follow-up study. PLoS One 2012; 7: e38639.
Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 2012; 33: 1203–14.
Cui Y, Liu B, Luo S, Zhen X, Fan M, Liu T, et al. Identification of Conversion from mild cognitive impairment to Alzheimer's disease using multivariate predictors. PLoS One 2011; 6: e21896.
Acknowledgements
Data collection and sharing for this paper was provided by the Alzheimer's disease Neuroimaging Initiative (ADNI, National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd; Astra Zeneca; Bayer Health Care; Bio Clinica, Inc; Biogen Idec Inc; Bristol-Myers Squibb Company; Eisai Inc; Elan Pharmaceuticals Inc; Eli Lilly and Company; F Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; GE Healthcare; Innogenetics, NV; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Medpace, Inc; Merck & Co, Inc; Meso Scale Diagnostics, LLC; Novartis Pharmaceuticals Corporation; Pfizer Inc; Servier; Synarc Inc; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego, USA. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles, USA. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Y., Li, L., Zhou, Ty. et al. Alzheimer's disease progression model based on integrated biomarkers and clinical measures. Acta Pharmacol Sin 35, 1111–1120 (2014). https://doi.org/10.1038/aps.2014.57
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2014.57
Keywords
This article is cited by
-
Comparative analysis of different brain regions using machine learning for prediction of EMCI and LMCI stages of Alzheimer’s disease
Multimedia Tools and Applications (2023)
-
Perillaldehyde improves cognitive function in vivo and in vitro by inhibiting neuronal damage via blocking TRPM2/NMDAR pathway
Chinese Medicine (2021)
-
Detection of gray matter microstructural changes in Alzheimer’s disease continuum using fiber orientation
BMC Neurology (2020)
-
Testing whether the progression of Alzheimer’s disease changes with the year of publication, additional design, and geographical area: a modeling analysis of literature aggregate data
Alzheimer's Research & Therapy (2020)
-
Realistic simulation of virtual multi-scale, multi-modal patient trajectories using Bayesian networks and sparse auto-encoders
Scientific Reports (2020)