Abstract
Aim:
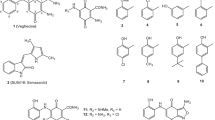
Targeting the VEGF/VEGF receptor (VEGFR) pathway has proved to be an effective antiangiogenic approach for cancer treatment. Here, we identified 6-((2-((3-acetamidophenyl)amino)pyrimidin-4-yl)oxy)-N-phenyl-1-naphthamide (designated herein as DW10075) as a novel and highly selective inhibitor of VEGFRs.
Methods:
In vitro tyrosine kinase activity was measured using ELISA, and intracellular signaling pathway proteins were detected by Western blot analysis. Endothelial cell proliferation was examined with CCK-8 assays, and tumor cell proliferation was determined with SRB assays. Cell migration, tube formation and rat aortic ring assays were used to detect antiangiogenic activity. Antitumor efficacy was further evaluated in U87-MG human glioblastoma xenograft tumors in nude mice receiving DW10075 (500 mg·kg−1·d−1, po) for two weeks.
Results:
Among a panel of 21 kinases tested, DW10075 selectively inhibited VEGFR-1, VEGFR-2 and VEGFR-3 (the IC50 values were 6.4, 0.69 and 5.5 nmol/L, respectively), but did not affect 18 other kinases including FGFR and PDGFR at 10 μmol/L. DW10075 significantly blocked VEGF-induced activation of VEGFR and its downstream signaling transduction in primary human umbilical vein endothelial cells (HUVECs), thus inhibited VEGF-induced HUVEC proliferation. DW10075 (1–100 nmol/L) dose-dependently inhibited VEGF-induced HUVEC migration and tube formation and suppressed angiogenesis in both the rat aortic ring model and the chicken chorioallantoic membrane model. Furthermore, DW10075 exhibited anti-proliferative activity against 22 different human cancer cell lines with IC50 values ranging from 2.2 μmol/L (for U87-MG human glioblastoma cells) to 22.2 μmol/L (for A375 melanoma cells). In U87-MG xenograft tumors in nude mice, oral administration of DW10075 significantly suppressed tumor growth, and reduced the expression of CD31 and Ki67 in the tumor tissues.
Conclusion:
DW10075 is a potent and highly selective inhibitor of VEGFR that deserves further development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weis SM, Cheresh DA . Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011; 17: 1359–70.
Potente M, Gerhardt H, Carmeliet P . Basic and therapeutic aspects of angiogenesis. Cell 2011; 146: 873–87.
Ferrara N, Gerber H-P, LeCouter J . The biology of VEGF and its receptors. Nat Med 2003; 9: 669–76.
Bergers G, Benjamin LE . Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–10.
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011; 91: 1071–121.
Willett CG, Boucher Y, Di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–7.
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007; 25: 1539–44.
Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL . Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol 2009; 27: 4966–72.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 2006; 355: 2542–50.
Chow LQ, Eckhardt SG . Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007; 25: 884–96.
Iyer R, Fetterly G, Lugade A, Thanavala Y . Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother 2010; 11: 1943–55.
Bukowski RM, Yasothan U, Kirkpatrick P . Pazopanib. Nat Rev Drug Discovery 2010; 9: 17–8.
Traynor K . Cabozantinib approved for advanced medullary thyroid cancer. Am J Health Syst Pharm 2013; 70: 88.
Andre T, Dumont SN . Regorafenib approved in Metastatic Colorectal cancer. Bull Cancer 2013; 100: 1027–9.
Ivy SP, Wick JY, Kaufman BM . An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol 2009; 6: 569–79.
Shepard DR, Garcia JA . Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert Rev Anticancer Ther 2009; 9: 795–805.
Kumar R, Crouthamel MC, Rominger DH, Gontarek RR, Tummino PJ, Levin RA, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer 2009; 101: 1717–23.
Bhargava P, Robinson MO . Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep 2011; 13: 103–11.
Spratlin J . Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep 2011; 13: 97–102.
Poole RM, Vaidya A . Ramucirumab: first global approval. Drugs 2014; 74: 1047–58.
Ho TH, Jonasch E . Axitinib in the treatment of metastatic renal cell carcinoma. Future Oncol 2011; 7: 1247–53.
Peng T, Wu JR, Tong LJ, Li MY, Chen F, Leng YX, et al. Identification of DW532 as a novel anti-tumor agent targeting both kinases and tubulin. Acta Pharmacol Sin 2014; 35: 916–28.
Chen LK, Qiang PF, Xu QP, Zhao YH, Dai F, Zhang L . Trans-3, 4, 5, 4′-tetramethoxystilbene, a resveratrol analog, potently inhibits angiogenesis in vitro and in vivo. Acta Pharmacol Sin 2013; 34: 1174–82.
Aplin AC, Nicosia RF . The rat aortic ring model of angiogenesis. Methods Mol Biol 2015; 1214: 255–64.
Rao N, Lee YF, Ge R . Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol Sin 2015; 36: 1177–90.
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 2008; 14: 7272–83.
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374–80.
Goel HL, Mercurio AM . VEGF targets the tumour cell. Nat Rev Cancer 2013; 13: 871–82.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No 81173080, 81321092 and 81374063), the National Key Technology R&D Program of the Ministry of Science and Technology of China (2012BAI27B02) and the Research Program of Qinghai province (No 2012-T-Y23).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, My., Lv, Yc., Tong, Lj. et al. DW10075, a novel selective and small-molecule inhibitor of VEGFR, exhibits antitumor activities both in vitro and in vivo. Acta Pharmacol Sin 37, 398–407 (2016). https://doi.org/10.1038/aps.2015.117
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.117
Keywords
This article is cited by
-
C11, a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor, suppresses breast cancer metastasis and angiogenesis
Acta Pharmacologica Sinica (2019)
-
Structure-Guided Design of C4-alkyl-1,4-dihydro-2H-pyrimido[4,5-d][1,3]oxazin-2-ones as Potent and Mutant-Selective Epidermal Growth Factor Receptor (EGFR) L858R/T790M Inhibitors
Scientific Reports (2017)