Abstract
Aim:
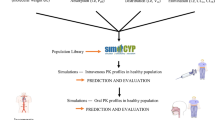
To evaluate the SimCYP simulator ethnicity-specific population model for predicting the pharmacokinetics of midazolam, a typical CYP3A4/5 substrate, in Chinese after oral administration.
Methods:
The physiologically based pharmacokinetic (PBPK) model for midazolam was developed using a SimCYP population-based simulator incorporating Chinese population demographic, physiological and enzyme data. A clinical trial was conducted in 40 Chinese subjects (the half was females) receiving a single oral dose of 15 mg midazolam. The subjects were separated into 4 groups based on age (20–50, 51–65, 66–75, and above 76 years), and the pharmacokinetics profiles of each age- and gender-group were determined, and the results were used to verify the PBPK model.
Results:
Following oral administration, the simulated profiles of midazolam plasma concentrations over time in virtual Chinese were in good agreement with the observed profiles, as were AUC and Cmax. Moreover, for subjects of varying ages (20–80 years), the ratios of predicted to observed clearances were between 0.86 and 1.12.
Conclusion:
The SimCYP PBPK model accurately predicted the pharmacokinetics of midazolam in Chinese from youth to old age. This study may provide novel insight into the prediction of CYP3A4/5-mediated pharmacokinetics in the Chinese population relative to Caucasians and other ethnic groups, which can support the rational design of bridging clinical trials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Evolving R&D for emerging markets. Nat Rev Drug Discov 2010; 9: 417–20.
Thiers FA, Sinskey AJ, Berndt ER . Trends in the globalization of clinical trials. Nat Rev Drug Discov 2008; 7: 13–4.
Karlberg JP . Globalization of sponsored clinical trials. Nat Rev Drug Discov 2009; 7: 458–60.
Yasuda SU, Zhang L, Huang SM . The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 2008; 84: 417–23.
Kim K, Johnson JA, Derendorf H . Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 2004; 44: 1083–105.
Rowland M, Peck C, Tucker G . Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 2011; 51: 45–73.
Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, et al. Model-based drug development. Clin Pharmacol Ther 2007; 82: 21–32.
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 2011; 89: 259–67.
Huang SM, Rowland M . The role of physiologically based pharmacokinetic modeling in regulatory review. Clin Pharmacol Ther 2012; 91: 542–9.
Zhao P, Rowland M, Huang SM . Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther 2012; 92: 17–20.
Barter ZE, Tucker GT, Rowland-Yeo K . Differences in cytochrome p450-mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modeling. Clin Pharmacokinet 2013; 52: 1085–100.
Li G, Yu G, Liu H, Zheng Q . Ethnic-specific in vitro–in vivo extrapolation and physiologically based pharmacokinetic approaches to predict cytochrome P450-mediated pharmacokinetics in Chinese population: opportunities and challenges. Clin Pharmacokinet 2014; 53: 197–202.
Lu Y, Yang J, Zhang H, Yang J . Prediction of warfarin maintenance dose in Han Chinese patients using a mechanistic model based on genetic and non-genetic factors. Clin Pharmacokinet 2013; 52: 567–81.
Wang B, Liu Z, Li D, Yang S, Hu J, Chen H, et al. Application of physiologically based pharmacokinetic modeling in the prediction of pharmacokinetics of bicyclol controlled-release formulation in human. Eur J Pharm Sci 2015; 77: 265–72.
Feng S, Cleary Y, Parrott N, Hu P, Weber C, Wang Y, et al. Evaluating a physiologically based pharmacokinetic model for prediction of omeprazole clearance and assessing ethnic sensitivity in CYP2C19 metabolic pathway. Eur J Clin Pharmacol 2015; 71: 617–24.
Zhu L, Yang J, Zhang Y, Wang Y, Zhang J, Zhao Y, et al. Prediction of pharmacokinetics and penetration of moxifloxacin in human with intra-abdominal infection based on extrapolated PBPK model. Korean J Physiol Pharmacol 2015; 19: 99–104.
Yu MM, Gao ZW, Chen XY, Zhong DF . Predicting pharmacokinetics of anti-cancer drug, famitinib in human using physiologically based pharmacokinetic model. Yao Xue Xue Bao 2014; 49: 1684–8.
Chen J, Liu D, Zheng X, Zhao Q, Jiang J, Hu P . Relative contributions of the major human CYP450 to the metabolism of icotinib and its implication in prediction of drug-drug interaction between icotinib and CYP3A4 inhibitors/inducers using physiologically based pharmacokinetic modeling. Expert Opin Drug Metab Toxicol 2015; 11: 857–68.
Jamei M, Dickinson GL, Rostami-Hodjegan A . A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: A tale of 'bottom-up' vs 'top-down' recognition of covariates. Drug Metab Pharmacokinet 2009; 24: 53–75.
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A . The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol 2009; 5: 211–23.
Kanto JH . Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy 1985; 5: 138–55.
Thummel K, O'shea D, Paine M, Shen D, Kunze K, Perkins J, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharm Ther 1996; 59: 491–502.
Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA . Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol 1989; 36: 89–96.
FDA Drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations (draft). 2012
Yang G, Fu Z, Chen X, Yuan H, Yang H, Huang Y, et al. Effects of the CYP oxidoreductase Ala503Val polymorphism on CYP3A activity in vivo: a randomized, open-label, crossover study in healthy Chinese men. Clin Ther 2011; 33: 2060–70.
Guo T, Mao GF, Xia DY, Su XY, Zhao LS . Pharmacokinetics of midazolam tablet in different Chinese ethnic groups. J Clin Pharm Ther 2011; 36: 406–11.
Shih PS, Huang JD . Pharmacokinetics of midazolam and 10-hydroxymidazolam in Chinese with different CYP3A5 genotypes. Drug Metab Dispos 2002; 30: 1491–6.
Luo G, Guenthner T, Gan LS, Humphreys WG . CYP3A4 induction by xenobiotics: biochemistry, experimental methods and impact on drug discovery and development. Curr Drug Metab 2004; 5: 483–505.
CHNS. China Health and Nutrition Survey. http://www.cpc.unc.edu/projects/china. Accessed 23 May 2013.
Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A . Prediction of in vivo drug clearance from in vitro data. I: Impact of inter-individual variability. Xenobiotica 2006; 36: 473–97.
Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997; 283: 1552–62.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J 2009; 11: 225–37.
Rodgers T, Rowland M . Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res 2007; 24: 918–33.
Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther 1999; 65: 630–9.
Platten HP, Schweizer E, Dilger K, Mikus G, Klotz U . Pharmacokinetics and the pharmacodynamic action of midazolam in young and elderly patients undergoing tooth extraction. Clin Pharmacol Ther 1998; 63: 552–60.
Tomoki N, Takashi M, Kazuo H . The effects of age and gender on the optimal premeditation dose of intramuscular midazolam. Anesth Analg 1998; 86: 1103–8.
Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI . Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology 1984; 61: 25–35.
Shu Y, Wang LS, Xiao WM, Wang W, Huang SL, Zhou HH . Probing CYP2C19 and CYP3A4 activities in Chinese liver microsomes by quantification of 5-hydroxyomeprazole andomeprazole sulphone. Acta Pharmacol Sin 2000; 21: 753–8.
Shu Y, Cheng ZN, Liu ZQ, Wang LS, Zhu B, Huang SL, et al. Interindividual variations in levels and activities of cytochrome P-450 in liver microsomes of Chinese subjects. Acta Pharmacol Sin 2001; 22: 283–8.
Rogers J, Rocci M, Haughey D, Bertino J . An evaluation of the suitability of IV midazolam as an in vivo marker for hepatic CYP3A activity. Clin Pharmacol Ther 2003; 273: 153–8.
Wilkinson G, Shand D . Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther 1975; 18: 377–90.
Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res 2004; 10: 8341–50.
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN . Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 1999; 37: 485–505.
Acknowledgements
This study was supported by the National Major Scientific and Technological Special Project: Innovative Drugs Development (2012–2015, No 2012ZX09303-006-002), and the PUMCH-Roche Quantitative Pharmacology Fellowship Program (2012–2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Hy., Chen, X., Jiang, J. et al. Evaluating a physiologically based pharmacokinetic model for predicting the pharmacokinetics of midazolam in Chinese after oral administration. Acta Pharmacol Sin 37, 276–284 (2016). https://doi.org/10.1038/aps.2015.122
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.122
Keywords
This article is cited by
-
Altered Bioavailability and Pharmacokinetics in Crohn’s Disease: Capturing Systems Parameters for PBPK to Assist with Predicting the Fate of Orally Administered Drugs
Clinical Pharmacokinetics (2022)
-
Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine
Journal of Pharmacokinetics and Pharmacodynamics (2016)