Abstract
Aim:
We have shown that low-dose gadolinium chloride (GdCl3) abolishes arachidonic acid (AA)-induced increase of cytoplasmic Ca2+, which is known to play a crucial role in myocardial ischemia/reperfusion (I/R) injury. The present study sought to determine whether low-dose GdCl3 pretreatment protected rat myocardium against I/R injury in vitro and in vivo.
Methods:
Cultured neonatal rat ventricular myocytes (NRVMs) were treated with GdCl3 or nifedipine, followed by exposure to anoxia/reoxygenation (A/R). Cell apoptosis was detected; the levels of related signaling molecules were assessed. SD rats were intravenously injected with GdCl3 or nifedipine. Thirty min after the administration the rats were subjected to LAD coronary artery ligation followed by reperfusion. Infarction size, the release of serum myocardial injury markers and AA were measured; cell apoptosis and related molecules were assessed.
Results:
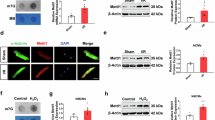
In A/R-treated NRVMs, pretreatment with GdCl3 (2.5, 5, 10 μmol/L) dose-dependently inhibited caspase-3 activation, death receptor-related molecules DR5/Fas/FADD/caspase-8 expression, cytochrome c release, AA release and sustained cytoplasmic Ca2+ increases induced by exogenous AA. In I/R-treated rats, pre-administration of GdCl3 (10 mg/kg) significantly reduced the infarct size, and the serum levels of CK-MB, cardiac troponin-I, LDH and AA. Pre-administration of GdCl3 also significantly decreased the number of apoptotic cells, caspase-3 activity, death receptor-related molecules (DR5/Fas/FADD) expression and cytochrome c release in heart tissues. The positive control drug nifedipine produced comparable cardioprotective effects in vitro and in vivo.
Conclusion:
Pretreatment with low-dose GdCl3 significantly attenuates I/R-induced myocardial apoptosis in rats by suppressing activation of both death receptor and mitochondria-mediated pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Frangogiannis NG, Smith CW, Entman ML . The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53: 31–47.
Sutton MG, Sharpe N . Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000; 101: 2981–8.
Mersmann J, Latsch K, Habeck K, Zacharowski K . Measure for ameasure-determination of infarct size in murine models of myocardial ischemia and reperfusion: a systematic review. Shock 2011; 35: 449–55.
Yellon DM, Hausenloy DJ . Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–35.
Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T . Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth 2012; 16: 123–32.
Gustafsson AB, Gottlieb RA . Mechanisms of apoptosis in the heart. J Clin Immunol 2003; 23: 447–59.
Liu H, Guo X, Chu Y, Lu S . Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene 2014; 545: 149–55.
Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, et al. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochim Biophys Acta 2014; 1842: 22–31.
Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I . Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol 2004; 43: 295–301.
Boisguerin P, Giorgi JM, Barrère-Lemaire S . CPP-conjugated anti-apoptotic peptides as therapeutic tools of ischemia-reperfusion injuries. Curr Pharm Des 2013; 19: 2970–8.
Dorn GW 2nd . Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res 2009; 81: 465–73.
Kawamura T, Hasegawa K, Morimoto T, Iwakura A, Nishina T, Nomoto T, et al. Down-regulation of endothelin-1 and alteration of apoptosis signaling following left ventricular volume reduction surgery in heart failure of adult rats. J Cardiovasc Pharmacol 2004; 44: S366–71.
Li D, Liu M, Tao TQ, Song DD, Liu XH, Shi DZ . Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem 2014; 34: 1413–26.
Stephanou A, Scarabelli TM, Brar BK, Nakanishi Y, Matsumura M, Knight RA, et al. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J Biol Chem 2001; 276: 28340–7.
Czerski L, Nunez G . Apoptosome formation and caspase activation: is it different in the heart. J Mol Cell Cardiol 2004; 37: 643–52.
Fang KM, Lee AS, Su MJ, Lin CL, Chien CL, Wu ML . Free fatty acids act as endogenous ionophores, resulting in Na+ and Ca2+ influx and myocyte apoptosis. Cardiovasc Res 2008; 78: 533–45
Maia RC, Culver CA, Laster SM . Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids. J Immunol 2006; 177: 6398–404.
Luo D, Broad LM, Bird GJ, Putney JW Jr . Mutual antagonism of calcium entry by capacitative and arachidonic acid-mediated Ca2+ entry pathways. J Biol Chem 2001; 276: 20186–9.
Luo D, Sun H, Lan X, Xiao R, Han Q . Direct coupling between AA-induced Ca2+ release and Ca2+ entry in HEK293 cells. Prostgland Other Mediat 2005; 75: 141–51.
Zheng Y, Zhang H, Yan X, Chen M, Qi T, Zhang L, et al. Protective effect of low dose gadolinium chloride against isoproterenol-induced myocardial injury in rat. Apoptosis 2015; 20: 1164–75.
Yan L, Zhu TB, Wang LS, Pan SY, Tao ZX, Yang Z, et al. Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep 2011; 38: 2695–701.
Gulati P, Muthuraman A, Jaggi AS, Singh N . Neuroprotective effect of gadolinium: a stretch-activated calcium channel blocker in mouse model of ischemia-reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol 2013; 386: 255–64
Wang B, Zhang Q, Zhu B, Cui Z, Zhou J . Protective effect of gadolinium chloride on early warm ischemia/reperfusion injury in rat bile duct during liver transplantation. PLoS One 2013; 8: e52743.
Strande JL, Routhu KV, Hsu A, Nicolosi AC, Baker JE . Gadolinium decreases inflammation related to myocardial ischemia and reperfusion injury. J Inflamm 2009; 6: 34.
Schneider L, Hackert T, Longerich T, Hartwig W, Fritz S, Krych R, et al. Effects of gadolinium chloride and glycine on hepatic and pancreatic tissue damage in alcoholic pancreatitis. Pancreas 2010; 39: 502–9.
Duan JL, Wang JW, Guan Y, Yin Y, Wei G, Cui J, et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin 2013; 34: 487–95.
Luo D, Yang D, Lan X, Li K, Li X, Chen J, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium 2008; 43: 165–74.
Chang R, Li Y, Yang X, Yue Y, Dou L, Wang Y, et al. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemia–reperfusion injury in rats. PloS One 2013; 8: e61590.
Zhang MQ, Zhang YL, Chen H, Tu JF, Shen Y, Guo JP, et al. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin 2013; 34: 1386–96.
Elsässer A, Suzuki K, Schaper J . Unresolved issues regarding the role of apoptosis of ischemic injury and heart failure. J Mol Cell Cardiol 2000; 32: 711–24.
Yousif MH, Benter IF, Roman RJ . Cytochrome P450 metabolites of arachidonic acid play a role in the enhanced cardiac dysfunction in diabetic rats following ischaemic reperfusion injury. Auton Autacoid Pharmacol 2009; 29: 33–41.
Gross GJ, Falck JR, Cross ER, Isbell M, Moore J, Nithipatikom K . Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 2005; 68: 18–25.
Liang Z, Liu LF, Yao TM, Huo Y, Han YL . Cardioprotective effects of Guanxinshutong (GXST) against myocardial ischemia/reperfusion injury in rats. J Geriatr Cardiol 2012; 9: 130–6.
Giakoustidis D, Papageorgiou G, Iliadis S, Giakoustidis A, Kostopoulou E, Kontos N, et al. The protective effect of alpha-tocopherol and GdCl3 against hepatic ischemia/reperfusion injury. Surg Today 2006; 36: 450–6.
Kim SJ, Kuklov A, Crystal GJ . In vivo gene delivery of XIAP protects against myocardial apoptosis and infarction following ischemia/reperfusion conscious rabbits. Life Sci 2011; 88: 572–7.
Scarabelli TM, Knight R, Stephanou A, Townsend P, Chen-Scarabelli C, Lawrence K, et al. Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol 2006; 31: 181–264.
Kristen AV, Ackermann K, Buss S, Lehmann L, Schnabel PA, Haunstetter A, et al. Inhibition of apoptosis by the intrinsic but not the extrinsic apoptotic pathway in myocardial ischemia-reperfusion. Cardiovasc Pathol 2013; 22: 280–6.
Voora D, Ortel TL, Lucas JE, Chi JT, Becker RC, Ginsburg GS . Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. J Thromb Thrombolysis 2012; 33: 246–57.
Fang KM, Chang WL, Wang SM, Su MJ, Wu ML . Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem 2008; 104: 1177–89.
Peter ME, Krammer PH . The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ 2003; 10: 26–35.
Nicolosi AC, Kwok CS, Logan B . Effects of gadolinium on regionally stunned myocardium: temporal considerations. J Surg Res 2007; 139: 286–91.
Bhuiyan MS, Fukunaga K . Inhibition of HtrA2/Omi ameliorates heart dysfunction following ischemia/reperfusion injury in rat heart in vivo. Eur J Pharmacol 2007; 557: 168–77.
Hao J, Kim HS, Choi W, Ha TS, Ahn HY, Kim CH . Mechanical stretch-induced protection against myocardial ischemia-reperfusion injury involves AMP-activated protein kinase. Korean J Physiol Pharmacol 2010; 14: 1–9.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No 81302777 and 81370339) and the Beijing Key Laboratory of Cardiovascular Diseases Related to Metabolic Disturbance (No Z13111000280000).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, M., Zheng, Yy., Song, Yt. et al. Pretreatment with low-dose gadolinium chloride attenuates myocardial ischemia/reperfusion injury in rats. Acta Pharmacol Sin 37, 453–462 (2016). https://doi.org/10.1038/aps.2015.156
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.156
Keywords
This article is cited by
-
Gadolinium and ruthenium red attenuate remote hind limb preconditioning-induced cardioprotection: possible role of TRP and especially TRPV channels
Naunyn-Schmiedeberg's Archives of Pharmacology (2016)