Abstract
Aim:
Oral risedronate is effective in the treatment of postmenopausal osteoporosis when administered daily, weekly, or monthly. In this 1-year, randomized, double-blind, multicenter study we compared the weekly 35-mg and daily 5-mg risedronate dosing regimens in the treatment of Chinese postmenopausal women with osteoporosis or osteopenia.
Methods:
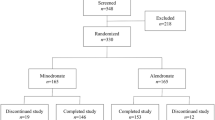
Postmenopausal women with primary osteoporosis or osteopenia were randomly assigned to the weekly group or daily group (n=145 for each) that received oral risedronate 35 mg once a week or 5 mg daily, respectively, for 1 year. The subjects' bone mineral densities (BMDs), bone turnover markers (P1NP and β-CTX), new vertebral fractures, and adverse events were assessed at baseline and during the treatments.
Results:
All subjects in the weekly group and 144 subjects in the daily group completed the study. The primary efficacy endpoint after 1 year, ie the mean percent changes in the lumbar spine BMD (95% CI) were 4.87% (3.92% to 5.81%) for the weekly group and 4.35% (3.31% to 5.39%) for the daily group. The incidences of clinical adverse events were 48.3% in the weekly group and 54.2% in the daily group.
Conclusion:
The weekly 35-mg and daily 5-mg risedronate dosing regimens during 1 year of follow-up show similar efficacy in improving BMDs and biochemical markers of bone turnover in Chinese postmenopausal women with osteoporosis or osteopenia. Moreover, the two dosing regimens exhibit similar safety and tolerability.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282: 1344–52.
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11: 83–91.
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344: 333–40.
Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 2002; 71: 103–11.
McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, et al. Efficacy and safety of risedronate 150-mg once a month in the treatment of postmenopausal osteoporosis: 2-year data. Osteoporos Int 2013; 24: 293–9.
Kanis JA . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368–81.
Yue H, He JW, Zhang H, Wang C, Hu WW, Gu JM, et al. Contribution of myostatin gene polymorphisms to normal variation in lean mass, fat mass and peak BMD in Chinese male offspring. Acta Pharmacol Sin 2012; 33: 660–7.
Genant HK, Wu CY, van Kuijk C, Nevitt MC . Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993; 8: 1137–48.
Zhang H, Huang QR, Gu JM, Hu WW, Liu YJ, Hu YQ, et al. Comparison of the effects of cholecalciferol and calcitriol on calcium metabolism and bone turnover in Chinese postmenopausal women with vitamin D insufficiency. Acta Pharmacol Sin 2012; 33: 490–5.
Thomson AB, Marshall JK, Hunt RH, Provenza JM, Lanza FL, Royer MG, et al. Forteen day endoscopy study comparing risedronate and alendronate in postmenopausal women stratified by Helicobacter pylori status. J Rheumatol 2002; 29: 1965–74.
Ralston SH, Kou TD, Wick-Urban B, Steinbuch M, Masud T . Risk of upper gastrointestinal tract events in risedronate users switched to alendronate. Calcif Tissue Int 2010; 87: 298–304.
Delmas PD, Benhamou CL, Man Z, Tlustochowicz W, Matzkin E, Eusebio R, et al. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int 2008; 19: 1039–45.
Acknowledgements
This study was sponsored by Kunming Jida Pharmaceutical Co, Ltd, Kunming, China.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Gu, Jm., Wang, L., Lin, H. et al. The efficacy and safety of weekly 35-mg risedronate dosing regimen for Chinese postmenopausal women with osteoporosis or osteopenia: 1-year data. Acta Pharmacol Sin 36, 841–846 (2015). https://doi.org/10.1038/aps.2015.30
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.30
Keywords
This article is cited by
-
Development of UV Spectrophotometric Methods for the Determination of Risedronate Sodium in Different Solutions
Journal of Applied Spectroscopy (2021)