Abstract
Aim:
Recent evidence suggests that the essential amino acid leucine may be involved in systemic cholesterol metabolism. In this study, we investigated the effects of leucine supplementation on the development of atherosclerosis in apoE null mice.
Methods:
ApoE null mice were fed with chow supplemented with leucine (1.5% w/v) in drinking water for 8 week. Aortic atherosclerotic lesions were examined using Oil Red O staining. Plasma lipoprotein-cholesterol levels were measured with fast protein liquid chromatography. Hepatic gene expression was detected using real-time PCR and Western blot analyses.
Results:
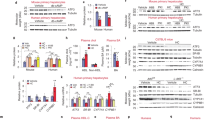
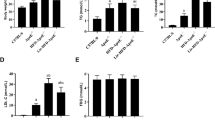
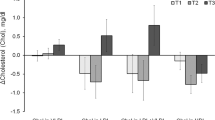
Leucine supplementation resulted in 57.6% reduction of aortic atherosclerotic lesion area in apoE null mice, accompanied by 41.2% decrease of serum LDL-C levels and 40.2% increase of serum HDL-C levels. The body weight, food intake and blood glucose level were not affected by leucine supplementation. Furthermore, leucine supplementation increased the expression of Abcg5 and Abcg8 (that were involved in hepatic cholesterol efflux) by 1.28- and 0.86-fold, respectively, and significantly increased their protein levels. Leucine supplementation also increased the expression of Srebf1, Scd1 and Pgc1b (that were involved in hepatic triglyceride metabolism) by 3.73-, 1.35- and 1.71-fold, respectively. Consequently, leucine supplementation resulted in 51.77% reduction of liver cholesterol content and 2.2-fold increase of liver triglyceride content. Additionally, leucine supplementation did not affect the serum levels of IL-6, IFN-γ, TNF-α, IL-10 and IL-12, but markedly decreased the serum level of MCP-1.
Conclusion:
Leucine supplementation effectively attenuates atherosclerosis in apoE null mice by improving the plasma lipid profile and reducing systemic inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB . Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986; 256: 2835–8.
Dresel HA, Friedrich EA, Otto I, Waldherr R, Schettler G . The low density lipoprotein and low density lipoprotein receptors and their possible importance in the pathogenesis of atherosclerosis. Arzneimittelforschung 1985; 35: 1936–40.
Wu G . Amino acids: metabolism, functions, and nutrition. Amino Acids 2009; 37: 1–17.
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH . Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007; 56: 1647–54.
Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010; 59: 17–25.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–26.
Kurano M, Hara M, Tsuneyama K, Okamoto K, Iso ON, Matsushima T, et al. Modulation of lipid metabolism with the overexpression of NPC1L1 in mouse liver. J Lipid Res 2012; 53: 2275–85.
Dai XY, Cai Y, Sun W, Ding Y, Wang W, Kong W, et al. Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc Res 2014; 101: 297–305.
Skov AR, Toubro S, Ronn B, Holm L, Astrup A . Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23: 528–36.
Parker B, Noakes M, Luscombe N, Clifton P . Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425–30.
Layman DK, Shiue H, Sather C, Erickson DJ, Baum J . Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr 2003; 133: 405–10.
Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr 2003; 133: 411–7.
Leenders M, van Loon LJ . Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr Rev; 69: 675–89.
Layman DK, Walker DA . Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr 2006; 136: 319S–23S.
Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J . Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr 2002; 132: 95–100.
Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, et al. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr 2006; 136: 529S–32S.
Gomes-Marcondes MC, Ventrucci G, Toledo MT, Cury L, Cooper JC . A leucine-supplemented diet improved protein content of skeletal muscle in young tumor-bearing rats. Braz J Med Biol Res 2003; 36: 1589–94.
Anthony JC, Anthony TG, Layman DK . Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr 1999; 129: 1102–6.
Nishitani S, Takehana K, Fujitani S, Sonaka I . Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol 2005; 288: G1292–300.
Guo K, Yu YH, Hou J, Zhang Y . Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010; 7: 57.
Hinault C, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E . Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J 2004; 18: 1894–6.
Guo F, Cavener DR . The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 2007; 5: 103–14.
Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes; 60: 746–56.
Nair KS, Short KR . Hormonal and signaling role of branched-chain amino acids. J Nutr 2005; 135: 1547S–52S.
Miller GJ, Miller NE . Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975; 1: 16–9.
Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, et al. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem 2001; 276: 27584–90.
Fitzgerald ML, Mujawar Z, Tamehiro N . ABC transporters, atheros-clerosis and inflammation. Atherosclerosis 2010; 211: 361–70.
Matsuo M . ATP-binding cassette proteins involved in glucose and lipid homeostasis. Biosci Biotechnol Biochem 2010; 74: 899–907.
Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest 2002; 110: 659–69.
Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ . Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem 2002; 277: 18793–800.
Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 2002; 110: 671–80.
Yu L, Gupta S, Xu F, Liverman AD, Moschetta A, Mangelsdorf DJ, et al. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J Biol Chem 2005; 280: 8742–7.
Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, et al. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 2004; 126: 290–300.
Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH . Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem 2003; 278: 15565–70.
Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, et al. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med 2004; 2: 5.
Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A 2002; 99: 16237–42.
Libby P . Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006; 83: 456S–60S.
Gewaltig MT, Kojda G . Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res 2002; 55: 250–60.
Lowenstein CJ . Beneficial effects of neuronal nitric oxide synthase in atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 1417–8.
Momi S, Monopoli A, Alberti PF, Falcinelli E, Corazzi T, Conti V, et al. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovasc Res 2012; 94: 428–38.
Muller G, Morawietz H . Nitric oxide, NAD(P)H oxidase, and atheros-clerosis. Antioxid Redox Signal 2009; 11: 1711–31.
Hayashi T, Iguchi A . Possibility of the regression of atherosclerosis through the prevention of endothelial senescence by the regulation of nitric oxide and free radical scavengers. Geriatr Gerontol Int 2010; 10: 115–30.
Li H, Forstermann U . Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr Pharm Des 2009; 15: 3133–45.
Acknowledgements
This study was supported by the National Basic Research Program of China (No 2011CB503904 and 2012CB518002), and the National Natural Science Foundation of China (No 31230035, 81270370, and 81470557).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Y., Dai, Xy., Zhou, Z. et al. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharmacol Sin 37, 196–203 (2016). https://doi.org/10.1038/aps.2015.88
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2015.88
Keywords
This article is cited by
-
Branched-chain amino acids in cardiovascular disease
Nature Reviews Cardiology (2023)
-
Adherence to aerobic training combined with high protein intake is associated with low blood pressure in Italian older adults: a cross-sectional study
Aging Clinical and Experimental Research (2023)