Abstract
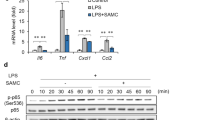
Recent studies show that Polydatin (PD) extracted from the roots of Polygonum cuspidatum Sieb, a widely used traditional Chinese remedies, possesses anti-inflammatory activity in several experimental models. In this study, we investigated the anti-inflammatory effects of PD on Staphylococcus aureus-induced mastitis in mice and elucidated the potential mechanisms. In mice with S aureus-induced mastitis, administration of PD (15, 30, 45 mg/kg, ip) or dexamethasone (Dex, 5 mg/kg, ip) significantly suppressed the infiltration of inflammatory cells, ameliorated the mammary structural damage, and inhibited the activity of myeloperoxidase, a biomarker of neutrophils accumulation. Furthermore, PD treatment dose-dependently decreased the levels of TNF-α, IL-1β, IL-6 and IL-8 in the mammary gland tissues. PD treatment also dose-dependently decreased the expression of TLR2, MyD88, IRAK1, IRAK4 and TRAF6 as well as the phosphorylation of TAK1, MKK3/6, p38 MAPK, IκB-α and NF-κB in the mammary gland tissues. In mouse mammary epithelial cells (mMECs) infected by S aureus in vitro, pretreatment with PD dose-dependently suppressed the upregulated pro-inflammatory cytokines and signaling proteins, and the nuclear translocation of NF-κB p65 and AP-1. A TLR2-neutralizing antibody mimicked PD in its suppression on S aureus-induced upregulation of MyD88, p-p38 and p-p65 levels in mMECs. PD (50, 100 μg/mL) affected neither the growth of S aureus in vitro, nor the viability of mMECs. In conclusion, PD does not exhibit antibacterial activity against S aureus, its therapeutic effects in mouse S aureus-induced mastitis depend on its ability to down-regulate pro-inflammatory cytokine levels via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fernández L, Arroyo R, Espinosa I, Marín M, Jiménez E, Rodríguez J . Probiotics for human lactational mastitis. Benef Microbes 2014; 5: 169–83.
Miao J, Zhang J, Ma Z, Zheng L . The role of NADPH oxidase in taurine attenuation of Streptococcus uberis-induced mastitis in rats. Int Immunopharmacol 2013; 16: 429–35.
Sears PM, McCarthy KK . Management and treatment of staphylococcal mastitis. Vet Clin North Am Food Anim Pract 2003; 19: 171–85.
Niebyl JR, Spence M, Parmley T . Sporadic (nonepidemic) puerperal mastitis. J Reprod Med 1978; 20: 97–100.
Twomey DP, Wheelock AI, Flynn J, Meaney WJ, Hill C, Ross RP . Protection against Staphylococcus aureus mastitis in dairy cows using a bismuth-based teat seal containing the bacteriocin, lacticin 3147. J Dairy Sci 2000; 83: 1981–8.
Elazar S, Gonen E, Livneh-Kol A, Rosenshine I, Shpigel NY . Neutrophil recruitment in endotoxin-induced murine mastitis is strictly dependent on mammary alveolar macrophages. Vet Res 2010; 41: 1–14.
Beutler BA . TLRs and innate immunity. Blood 2009; 113: 1399–407.
Medzhitov R . Recognition of microorganisms and activation of the immune response. Nature 2007; 449: 819–26.
Takeda K, Akira S . TLR signaling pathways. Semin Immunol 2004; 16: 3–9.
Takeuchi O, Akira S . Pattern recognition receptors and inflammation. Cell 2010; 140: 805–20.
Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002; 420: 324–9.
O'Neill LA . The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev 2008; 226: 10–8.
Akira S, Uematsu S, Takeuchi O . Pathogen recognition and innate immunity. Cell 2006; 124: 783–801.
Janssens S, Beyaert R . A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 2002; 27: 474–82.
Carneiro DMVF, Domingues PF, Vaz AK . Innate immunity of the bovine mammary gland: response to infection. Ciência Rural 2009; 39: 1934–43.
Xing WW, Wu JZ, Jia M, Du J, Zhang H, Qin LP . Effects of polydatin from polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed Pharmacother 2009; 63: 457–62.
Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L, et al. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol 2013; 52: 28–35.
Jiang X, Liu W, Deng J, Lan L, Xue X, Zhang C, et al. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med 2013; 60: 292–9.
Guo MY, Li WY, Zhang Z, Qiu C, Li C, Deng G . Betulin suppresses S aureus-induced mammary gland inflammatory injury by regulating PPAR-γ in mice. Int Immunopharmacol 2015; 29: 824–31.
Li T, Liu Y, Li G, Wang X, Zeng Z, Cai S, et al. Polydatin attenuates ipopolysaccharide-induced acute lung injury in rats. Int J Clin Exp Pathol 2014; 7: 8401.
Hu X, Fu Y, Tian Y, Zhang Z, Zhang W, Gao X, et al. The anti-inflammatory effect of TR6 on LPS-induced mastitis in mice. Int Immunopharmacol 2016; 30: 150–6.
Takeuchi O, Hoshino K, Akira S . Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000; 165: 5392–6.
Sheu F, Chien PJ, Hsieh KY, Chin KL, Huang WT, Tsao CY, et al. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (bitter mushroom) that exhibits TLR2-dependent NF-κB activation and M1 polarization within murine macrophages. J Agric Food Chem 2009; 57: 4130–41.
Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, et al. Multiwalled carbon nanotubes activate NF-kappaB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis 2010; 15: 1507–16.
Lou T, Jiang W, Xu D, Chen T, Fu Y . Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation 2015; 38: 1213–20.
Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu DX, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol Sin 2011; 32: 1364–72.
Akira S, Hirano T, Taga T, Kishimoto T . Biology of multifunctional cytokines: IL 6 and related molecules (IL-1 and TNF). FASEB J 1990; 4: 2860–7.
Yang W, Zerbe H, Petzl W, Brunner RM, Günther J, Draing C, et al. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E coli, but S aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol Immunol 2008; 45: 1385–97.
Basset A, Zhang F, Benes C, Sayeed S, Herd M, Thompson C, et al. Toll-like receptor (TLR) 2 mediates inflammatory responses to oligomerized RrgA pneumococcal pilus type 1 protein. J Biol Chem 2013; 288: 2665–75.
Akira S, Takeda K . Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499–511.
Janssens S, Burns K, Tschopp J, Beyaert R . Regulation of interleukin-1 and lipopolysaccharide-induced NF-κB activation by alternative splicing of MyD88. Curr Biol 2002; 12: 467–71.
Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X . IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFκB. J Biol Chem 2001; 276: 41661–7.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J-i, Chen ZJ . TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001; 412: 346–51.
Pang HY, Liu G, Liu GT . Compound FLZ inhibits lipopolysaccharide-induced inflammatory effects via down-regulation of the TAK-IKK and TAK-JNK/p38MAPK pathways in RAW264.7 macrophages. Acta Pharmacol Sin 2009; 30: 209–18.
Wang T, Song X, Zhang Z, Guo M, Jiang H, Wang W, et al. Stevioside inhibits inflammation and apoptosis by regulating TLR2 and TLR2-related proteins in S aureus-infected mouse mammary epithelial cells. Int Immunopharmacol 2014; 22: 192–9.
Alva-Murillo N, Medina-Estrada I, Báez-Magaña M, Ochoa-Zarzosa A, López-Meza JE . The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol Immunol 2015; 68: 445–55.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No 31272631 and 31472254).
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Jiang, Kf., Zhao, G., Deng, Gz. et al. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol Sin 38, 211–222 (2017). https://doi.org/10.1038/aps.2016.123
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.123
Keywords
This article is cited by
-
RNA stability: a novel perspective on gene regulatory networks in bovine mastitis
BMC Genomics (2025)
-
Profile of circular RNAs in bovine mammary tissues infected with Staphylococcus aureus
Archives of Microbiology (2025)
-
MicroRNA expression profiling of ovine epithelial cells stimulated with the Staphylococcus aureus in vitro
Mammalian Genome (2024)
-
Improvement of vaginal probiotics Lactobacillus crispatus on intrauterine adhesion in mice model and in clinical practice
BMC Microbiology (2023)
-
Integrated analysis of inflammatory mRNAs, miRNAs, and lncRNAs elucidates the molecular interactome behind bovine mastitis
Scientific Reports (2023)