Abstract
Aim:
Our preliminary results show that huperzine A, an acetylcholinesterase inhibitor used to treat Alzheimer's disease (AD) patients in China, exhibits different pharmacokinetic features in elderly and young healthy subjects. However, its pharmacokinetic data in elderly subjects remains unavailable to date. Thus, we developed a population pharmacokinetic (PPK) model of huperzine A in elderly Chinese people, and identified the covariate affecting its pharmacokinetics for optimal individual administration.
Methods:
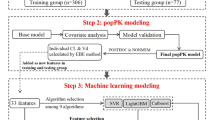
A total of 341 serum huperzine A concentration records was obtained from 2 completed clinical trials (14 elderly healthy subjects in a phase I pharmacokinetic study; 35 elderly AD patients in a phase II study). Population pharmacokinetic analysis was performed using the non-linear mixed-effect modeling software Phoenix NLME1.1.1. The effects of age, gender, body weight, height, creatinine, endogenous creatinine clearance rate as well as drugs administered concomitantly were analyzed. Bootstrap and visual predictive checks were used simultaneously to validate the final population pharmacokinetics models.
Results:
The plasma concentration-time profile of huperzine A was best described by a one-compartment model with first-order absorption and elimination. Age was identified as the covariate having significant influence on huperzine A clearance. The final PPK model of huperzine A was: CL (L/h)=2.4649*(age/86)(−3.3856), Ka=0.6750 h−1, V (L)=104.216. The final PPK model was demonstrated to be suitable and effective by the bootstrap and visual predictive checks.
Conclusion:
A PPK model of huperzine A in elderly Chinese subjects is established, which can be used to predict PPK parameters of huperzine A in the treatment of elderly AD patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lunardi P, Nardin P, Guerra MC, Abib R, Leite MC, Goncalves CA . Huperzine A, but not tacrine, stimulates S100B secretion in astrocyte cultures. Life Sci 2013; 92: 701–7.
Hu NH, Shi H, Chen GS . Clinical effect of huperzine A microemulsion cataplasm to Alzheimer patients by Governor vessel acu-points. Clin J Mod Appl Pharm 2014; 31: 581–3.
Shi CC, Yu F . Meta-analysis of efficacy and safety of huperzine A for treatment of Alzheimer's disease. Chin J New Drugs Clin Rem 2012; 31: 737–42.
Clinical study of huperzine A on patients with mild and moderate Alzheimer disease. Practical Pharmacy and Clinical Remedies 2013; 16: 2.
Zhang Z, Wang X, Chen Q, Shu L, Wang J, Shan G . Clinical efficacy and safety of huperzine Alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Zhonghua Yi Xue Za Zhi 2002; 82: 941–4.
Zhang ML, Yao JJ, Bi P, Ni SQ . Evaluation of clinical effect and safety of huperzine A in treating 52 Alzheimer's disease. Chin J New Drugs Clin Rem 2006; 25: 693–5.
Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011; 76: 1389–94.
Li YX, Zhang RQ, Li CR, Jiang XH . Pharmacokinetics of huperzine A following oral administration to human volunteers. Eur J Drug Metab Pharmacokinet 2007; 32: 183–7.
Qian BC, Wang M, Zhou ZF, Chen K, Zhou RR, Chen GS . Pharmacokinetics of tablet huperzine A in six volunteers. Acta Pharmacol Sin 1995; 16: 396–8.
Li W, Li J, Hu Q . Determination of huperzine A in human plasma by liquid chromatography-electrospray tandem mass spectrometry: application to a bioequivalence study on Chinese volunteers. Biomed Chromatogr 2008; 22: 354–60.
Liu GY, Wang W, MQ Z . Quantitation of huperzine A in human plasma by LC-MS/MS for elderly population. Clin J New Drugs Clin Remedies 2012; 31: 728–31.
Acknowledgements
We gratefully thank Jun-jie DING at Children's Hospital of Fudan University for technical assistance in PPK model building.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, L., Qu, Y., Yan, J. et al. Population pharmacokinetic modeling and simulation of huperzine A in elderly Chinese subjects. Acta Pharmacol Sin 37, 994–1001 (2016). https://doi.org/10.1038/aps.2016.24
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.24
Keywords
This article is cited by
-
Huperzine A ameliorates obesity-related cognitive performance impairments involving neuronal insulin signaling pathway in mice
Acta Pharmacologica Sinica (2020)