Abstract
Aim:
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a free radical scavenger that has shown potent antioxidant, anti-inflammatory and neuroprotective effects in variety of disease models. In this study, we investigated whether edaravone produced neuroprotective actions in an infant mouse model of pneumococcal meningitis.
Methods:
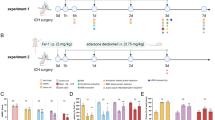
C57BL/6 mice were infected on postnatal d 11 by intracisternal injection of a certain inoculum of Streptococcus pneumoniae. The mice received intracisternal injection of 10 μL of saline containing edaravone (3 mg/kg) once a day for 7 d. The severity of pneumococcal meningitis was assessed with a clinical score. In mice with severe meningitis, the survival rate from the time of infection to d 8 after infection was analyzed using Kaplan-Meier curves. In mice with mild meningitis, the CSF inflammation and cytokine levels in the hippocampus were analyzed d 7 after infection, and the clinical neurological deficit score was evaluated using a neurological scoring system d 14 after infection. The nuclear factor (erythroid-derived 2)-like 2 knockout (Nrf2 KO) mice and heme oxygenase-1 knockout (HO-1 KO) mice were used to confirm the involvement of Nrf2/HO-1 pathway in the neuroprotective actions of edaravone.
Results:
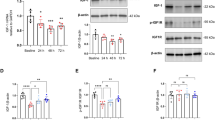
In mice with severe meningitis, edaravone treatment significantly increased the survival rate (76.4%) compared with the meningitis model group (32.2%). In mice with mild meningitis, edaravone treatment significantly decreased the number of leukocytes and TNF- levels in CSF, as well as the neuronal apoptosis and protein levels of HMGB1 and iNOS in the hippocampus, but did not affect the high levels of IL-10 and IL-6 in the hippocampus. Moreover, edaravone treatment significantly improved the neurological function of mice with mild meningitis. In Nrf2 KO or HO-1 KO mice with the meningitis, edaravone treatment was no longer effective in improving the survival rate of the mice with severe meningitis (20.2% and 53.6%, respectively), nor it affected the protein levels of HMGB1 and iNOS in the hippocampus of the mice with mild meningitis.
Conclusion:
Edaravone produces neuroprotective actions in a mouse model of pneumococcal meningitis by reducing neuronal apoptosis and HMGB1 and iNOS expression in the hippocampus via the Nrf2/HO-1 pathway. Thus, edaravone may be a promising agent for the treatment of bacterial meningitis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kim KS . Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci 2003; 4: 376–85.
Mook-Kanamori BB, Madelijn G, Tom VDP, Diederik VDB . Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 2011; 24: 721–36.
Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Ruth L, Hadler JL, et al. Bacterial meningitis in the United States, 1998-2007. New Engl J Med 2011; 364: 2016–25.
Aruna C, Hadley H, Derek M, Mathuram S . Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J 2011; 30: 3–6.
Grimwood K, Anderson P, Anderson V, Tan L, Nolan T . Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Arch Dis Child 2000; 83: 111–6.
Richard . Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ 2001; 323: 533–6.
Wellmer A, Noeske C, Gerber J, Munzel U, Nau R . Spatial memory and learning deficits after experimental pneumococcal meningitis in mice. Neurosci Lett 2000; 296: 137–40.
Loeffler JM, Ringer R, Hablützel M, Täuber MG, Leib SL . The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J Infect Dis 2001; 183: 247–52.
Zysk G, Brück W, Gerber J, Brück Y, Prange HW, Nau R . Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol 1996; 55: 722–8.
Christian S, Christian B, Helle Bossen K, Susanne S . Differences in survival, brain damage, and cerebrospinal fluid cytokine kinetics due to meningitis caused by 3 different Streptococcus pneumoniae serotypes: evaluation in humans and in 2 experimental models. J Infect Dis 2004; 190: 1212–20.
Coutinho LG, Christen S, Bellac CL, Fontes FL, Souza FR, Grandgirard D, et al. The kynurenine pathway is involved in bacterial meningitis. J Neuroinflammation 2014; 11: 169.
Tunkel AR, Scheld WM . Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev 1993; 6: 118–36.
Hajime K, Rock KL . How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8: 279–89.
Bianchi ME . DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81: 1–5.
Andersson U, Tracey KJ . HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011; 29: 139–62.
Christopher HH, Michael W, Barbara A, Sven H, Hans HC, Matthias K, et al. High mobility group box 1 prolongs inflammation and worsens disease in pneumococcal meningitis. Brain 2013; 136: 1746–59.
Boje KM . Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis. Brain Res 1996; 720: 75–83.
Uwe K, Andrea B, Robert P, Karl F, Adriano F, Pfister HW . Experimental pneumococcal meningitis: Cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol 1995; 37: 313–23.
Kolls JK . Oxidative stress in sepsis: a redox redux. J Clin Invest 2006; 116: 860–3.
Barichello T, Petronilho FC, Silva GZ, Souza B, Savi GD, Feier G, et al. Oxidative damage in the rat hippocampus and cortex after meningitis induced by Streptococcus pneumoniae. BMC Proceed 2008; 2: P6.
Tatiana B, Santos ALB, Savi GD, Generoso JS, Paola O, Michelon CM, et al. Antioxidant treatment prevents cognitive impairment and oxidative damage in pneumococcal meningitis survivor rats. Metab Brain Dis 2012; 27: 587–93.
Matthias K, Uwe K, Hans-Walter P . Oxidative stress in pneumococcal meningitis: a future target for adjunctive therapy? Prog Neurobiol 2006; 80: 269–80.
Watanabe T, Tahara M, Todo S . The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther 2008; 26: 101–14.
Arumugam S, Thandavarayan RA, Veeraveedu PT, Nakamura T, Arozal W, Sari FR, et al. Beneficial effects of edaravone, a novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol Med 2012; 16: 2176–85.
Zhang N, Kominekobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T . Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 2005; 36: 2220–5.
Group EAIS . Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis 2003; 15: 222–9.
Hiroshi Y, Hidekatsu Y, Yoshihisa N, Kayoko FS, Nobuyuki F, Norio T . Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev 2006; 12: 9–20.
Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, et al. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke 2008; 39: 463–9.
Yun Y, Hao Z, Rangarajan P, Ling EA, Wu C . Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia. BMC Neurosci 2014; 15: 125.
Grandgirard D, Steiner O, Täuber MG, Leib SL . An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol 2007; 114: 609–17.
Barichello T, Generoso JS, Simões LR, Elias SG, Tashiro MH, Dominguini D, et al. Inhibition of indoleamine 2,3-dioxygenase prevented cognitive impairment in adult Wistar rats subjected to pneumococcal meningitis. Transl Res 2013; 162: 390–7.
Wijsman JH, Jonker RR, Keijzer R, van de Velde CJ, Cornelisse CJ, van Dierendonck JH . A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem 1993; 41: 7–12.
Bifrare YD, Gianinazzi C, Imboden H, Leib SL, Täuber MG . Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 2003; 13: 481–8.
Spranger M, Schwab S, Krempien S, Winterholler M, Steiner T, Hacke W . Excess glutamate levels in the cerebrospinal fluid predict clinical outcome of bacterial meningitis. Arch Neurol 1996; 53: 992–6.
Park EJ, Jang HJ, Tsoyi K, Kim YM, Park SW, Kim HJ, et al. The heme oxygenase-1 inducer THI-56 negatively regulates iNOS expression and HMGB1 release in LPS-activated RAW 264.7 cells and CLP-induced septic mice. PLoS One 2013; 8: e76293.
Kim YM, Kim HJ, Chang KC . Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int Immunopharmacol 2015; 26: 112–8.
Barichello T, Savi GD, Panatto AP, Generoso JS, Cipriano AL, Rezin GT, et al. Effect of new compound with antioxidant potential in the energy metabolism of adults rats after pneumococcal meningitis. Am J Pharmacol Toxicol 2012; 7: 49–61.
Aycicek A, Iscan A, Erel O, Akcali M, Selek S . Total antioxidant/oxidant status in meningism and meningitis. Pediatr Neurol 2006; 35: 382–6.
Gu XL, Gao Y, Sun T, Zhu DY . Effects of edaravone on score of neurological behavior,cerebral ischemia areas and amount of bleeding after cerebral hemorrhage in rats. Chin J New Drugs Clin Remedies 2006; 25: 358–61.
Ostergaard C, Yieng-Kow RV, Benfield T, Frimodt-Møller N, Espersen F, Lundgren JD . Inhibition of leukocyte entry into the brain by the selectin blocker fucoidin decreases interleukin-1 (IL-1) levels but increases IL-8 levels in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect Immun 2000; 68: 3153–7.
Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D . Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 2011; 24: 557–91.
Prasad R, Kapoor R, Srivastava R, Mishra OP, Singh TB . Cerebrospinal fluid TNF-α, IL-6, and IL-8 in children with bacterial meningitis. Pediatr Neurol 2014; 50: 60–5.
Fassbender K, Schminke U, Ries S, Ragoschke A, Kischka U, Fatar M, et al. Endothelial-derived adhesion molecules in bacterial meningitis: association to cytokine release and intrathecal leukocyte-recruitment. J Neuroimmunol 1997; 74: 130–4.
Zwijnenburg PJ, van der Poll T, Florquin S, Roord JJ, van Furth AM . Interleukin-10 negatively regulates local cytokine and chemokine production but does not influence antibacterial host defense during murine pneumococcal meningitis. Infect Immun 2003; 71: 2276–9.
Leib SL, Leppert D, Clements J, Täuber MG . Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 2000; 68: 615–20.
Kikuta M, Shiba T, Yoneyama M, Kawada K, Yamaguchi T, Hinoi E, et al. In vivo and in vitro treatment with edaravone promotes proliferation of neural progenitor cells generated following neuronal loss in the mouse dentate gyrus. J Pharmacol Sci 2013; 121: 74–83.
Asano T, Ichiki K, Koizumi S, Kaizu K, Hatori T, Mashiko K, et al. High mobility group box 1 in cerebrospinal fluid from several neurological diseases at early time points. Int J Neurosci 2011; 121: 480–4.
Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, et al. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med 2008; 36: 291–5.
Kikuchi K, Kawahara K, Tancharoen S, Matsuda F, Morimoto Y, Ito T, et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther 2009; 329: 865–74.
Licinio J, Prolo P, Mccann SM, Wong ML . Brain iNOS: current understanding and clinical implications. Mol Med Today 1999; 5: 225–32.
Suzuki Y, Fujii S, Tominaga T, Yoshimoto T, Fujii S, Akaike T, et al. Direct evidence of in vivo nitric oxide production and inducible nitric oxide synthase mRNA expression in the brain of living rat during experimental meningitis. J Cereb Blood Flow Metab 1999; 19: 1175–8.
Kornelisse RF, Hoekman K, Visser JJ, Hop WC, Huijmans JG, van der Straaten PJ, et al. The role of nitric oxide in bacterial meningitis in children. J Infect Dis 1996; 174: 120–6.
Li B . The effect of edaravone on serum NO, NOS, MDA and SOD in patients with acute cerebral infarction. Chin J Arteriosclerosis 2010.
Zwijnenburg PJ, van der Poll T, Florquin S, Akira S, Takeda K, Roord JJ, et al. Interleukin-18 gene-deficient mice show enhanced defense and reduced inflammation during pneumococcal meningitis. J Neuroimmunol 2003; 138: 31–7.
Gupte AA, Lyon CJ, Hsueh WA . Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr Diab Rep 2013; 13: 362–71.
Stocker R, Yamamoto Y, Mcdonagh AF, Glazer AN, Ames BN . Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043–6.
Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2005; 289: L1131–7.
Wiesel P, Patel AP, Difonzo N, Marria PB, Sim CU, Pellacani A, et al. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 2000; 102: 3015–22.
Kanwar YS . Heme oxygenase-1 in renal injury: conclusions of studies in humans and animal models. Kidney Int 2001; 59: 378–9.
Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992; 90: 267–70.
Kim HS, Park EJ, Sang WP, Kim HJ, Chang KC . A tetrahydroisoquinoline alkaloid THI-28 reduces LPS-induced HMGB1 and diminishes organ injury in septic mice through p38 and PI3K/Nrf2/HO-1 signals. Int Immunopharmacol 2013; 17: 684–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Ma, Qq., Yan, Y. et al. Edaravone attenuates hippocampal damage in an infant mouse model of pneumococcal meningitis by reducing HMGB1 and iNOS expression via the Nrf2/HO-1 pathway. Acta Pharmacol Sin 37, 1298–1306 (2016). https://doi.org/10.1038/aps.2016.71
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.71
Keywords
This article is cited by
-
Edaravone Confers Neuroprotective, Anti-inflammatory, and Antioxidant Effects on the Fetal Brain of a Placental-ischemia Mouse Model
Journal of Neuroimmune Pharmacology (2023)
-
Ulinastatin Attenuates LPS-Induced Inflammation and Inhibits Endoplasmic Reticulum Stress–Induced Apoptosis in Renal Tubular Epithelial Cells via Regulation of the TLR4/NF-κB and Nrf2/HO-1 Pathways
Inflammation (2021)
-
Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway
Acta Pharmacologica Sinica (2018)
-
Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling
Acta Pharmacologica Sinica (2018)