Abstract
Aim:
Accumulation of α-synuclein (α-syn) in the brain is a characteristic of Parkinson's disease (PD). In this study, we investigated whether treatment with tunicamycin, an endoplasmic reticulum (ER) stress inducer, led to the accumulation of α-syn in PC12 cells, and where α-syn protein was accumulated, and finally, whether bibenzyl compound 20c, a novel compound isolated from Gastrodia elata (Tian ma), could alleviate the accumulation of α-syn and ER stress activation in tunicamycin-treated PC12 cells.
Methods:
PC12 cells were treated with tunicamycin for different time (6 h, 12 h, 24 h, 48 h). Cell viability was determined by a MTT assay. Subcellular fractions of ER and mitochondria were extracted with the Tissue Endoplasmic reticulum Isolation Kit. The levels of α-syn protein and ER-stress-associated downstream chaperones were detected using Western blots and immunofluorescence.
Results:
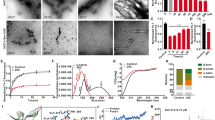
Treatment of PC12 cells with tunicamycin (0.5–10 μg/mL) dose-dependently increased the accumulation of α-syn monomer (19 kDa) and oligomer (55 kDa), and decreased the cell viability. Accumulation of the two forms of α-syn was observed in both the ER and mitochondria with increasing treatment time. Co-treatment with 20c (10−5 mol/L) significantly increased the viability of tunicamycin-treated cells, reduced the level of α-syn protein and suppressed ER stress activation in the cells, evidenced by the reductions in phosphorylation of eIF2α and expression of spliced ATF6 and XBP1.
Conclusion:
Tunicamycin treatment caused accumulation of α-syn monomer and oligomer in PC12 cells. Bibenzyl compound 20c reduces the accumulation of α-syn and inhibits the activation of ER stress, which protected PC12 cells against the toxicity induced by tunicamycin.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jiang P, Gan M, Ebrahim AS, Lin WL, Melrose HL, Yen SH . ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener 2010; 5: 56.
Raiss CC, Braun TS, Konings IB, Grabmayr H, Hassink GC, Sidhu A, et al. Functionally different alpha-synuclein inclusions yield insight into Parkinson's disease pathology. Sci Rep 2016; 6:23116.
Mercado G, Valdes P, Hetz C . An ERcentric view of Parkinson's disease. Trends Mol Med 2013; 19: 165–75.
Soto C . Transmissible proteins: expanding the prion heresy. Cell 2012; 149: 968–77.
Rutkowski DT, Kaufman RJ . A trip to the ER: coping with stress. Trends Cell Biol 2004; 14: 20–8.
Kim I, Xu W, Reed JC . Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008; 7: 1013–30.
Halliday M, Mallucci GR . Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology 2014; 76: 169–74.
Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ . Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 2013; 12: 105–18.
Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA . Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal 2015; 8: ra45.
Parkkinen L, Pirttila T, Alafuzoff I . Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 2008; 115: 399–407.
Ng CF, Ko CH, Koon CM, Chin WC, Themis Kwong HC, Lo AW, et al. The aqueous extract of rhizome of Gastrodia elata Blume attenuates locomotor defect and inflammation after traumatic brain injury in rats. J Ethnopharmacol 2016; 185: 87–95.
Manavalan A, Ramachandran U, Sundaramurthi H, Mishra M, Sze SK, Hu JM, et al. Gastrodia elata Blume (tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol 2012; 3: 219–41.
Ramachandran U, Manavalan A, Sundaramurthi H, Sze SK, Feng ZW, Hu JM, et al. Tianma modulates proteins with various neuro-regenerative modalities in differentiated human neuronal SH-SY5Y cells. Neurochem Int 2012; 60: 827–36.
Kogel D, Schomburg R, Schurmann T, Reimertz C, Konig HG, Poppe M, et al. The amyloid precursor protein protects PC12 cells against endoplasmic reticulum stress-induced apoptosis. J Neurochem 2003; 87: 248–56.
Zhou T, Zu G, Zhang X, Wang X, Li S, Gong X, et al. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology 2016; 101: 480–9.
Dong G, Chen T, Ren X, Zhang Z, Huang W, Liu L, et al. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016; 26: 7–18.
Ma KL, Song LK, Yuan YH, Zhang Y, Han N, Gao K, et al. The nuclear accumulation of alpha-synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology 2014; 82: 132–42.
Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, et al. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci 2012; 32: 3306–20.
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 2006; 313: 324–8.
Bao XQ, Wang XL, Zhang D . FLZ attenuates alpha-synuclein-induced neurotoxicity by activating heat shock protein 70. Mol Neurobiol 2016. DOI: 10.1007/s12035-015-9572-9.
Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D . alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A 2016; 113: 1931–6.
Tsigelny IF, Sharikov Y, Kouznetsova VL, Greenberg JP, Wrasidlo W, Overk C, et al. Molecular determinants of alpha-synuclein mutants' oligomerization and membrane interactions. ACS Chem Neurosci 2015; 6: 403–16.
Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, et al. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem 2008; 283: 23179–88.
Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 2005; 14: 3801–11.
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 2006; 4: e374.
Ma Y, Brewer JW, Diehl JA, Hendershot LM . Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 2002; 318: 1351–65.
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K . Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 2002; 366: 585–94.
Lindholm D, Wootz H, Korhonen L . ER stress and neurodegenerative diseases. Cell Death Differ 2006; 13: 385–92.
Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M, et al. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron 2010; 68: 865–78.
Belal C, Ameli NJ, El Kommos A, Bezalel S, Al'Khafaji AM, Mughal MR, et al. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant alpha-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum Mol Genet 2012; 21: 963–77.
Credle JJ, Forcelli PA, Delannoy M, Oaks AW, Permaul E, Berry DL, et al. alpha-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson's disease. Neurobiol Dis 2015; 76: 112–25.
Bir A, Sen O, Anand S, Khemka VK, Banerjee P, Cappai R, et al. alpha-Synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: implications in the pathogenesis of Parkinson's disease. J Neurochem 2014; 131: 868–77.
Chinta SJ, Mallajosyula JK, Rane A, Andersen JK . Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 2010; 486: 235–9.
Hetz C, Mollereau B . Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 2014; 15: 233–49.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81274122, 81373997, 81273629, 81473376, U1402221, and 81573640), the National Mega-project for Innovative Drugs (No 2012ZX09301002-004), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (No IRT1007), Beijing Natural Science Foundation (No 7131013 and 7161011), and the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No BZ0150).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mou, Z., Yuan, Yh., Lou, Yx. et al. Bibenzyl compound 20c protects against endoplasmic reticulum stress in tunicamycin-treated PC12 cells in vitro. Acta Pharmacol Sin 37, 1525–1533 (2016). https://doi.org/10.1038/aps.2016.75
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2016.75
Keywords
This article is cited by
-
Deficiency of RAB39B Activates ER Stress-Induced Pro-apoptotic Pathway and Causes Mitochondrial Dysfunction and Oxidative Stress in Dopaminergic Neurons by Impairing Autophagy and Upregulating α-Synuclein
Molecular Neurobiology (2023)
-
Gastrodin Derivatives from Gastrodia elata
Natural Products and Bioprospecting (2019)
-
Seipin deficiency in mice causes loss of dopaminergic neurons via aggregation and phosphorylation of α-synuclein and neuroinflammation
Cell Death & Disease (2018)