Abstract
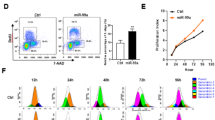
Cell-derived exosomes (EXs) can modulate target cell differentiation via microRNAs (miRs) that they carried. Previous studies have shown that miR126 is highly expressed in hematopoietic stem cells (HSCs) and plays a role in hematopoiesis via modulating the Notch pathway that participates in progenitors' cell fate decisions. In this study we investigated whether HSC-derived EXs (HSC-EXs) could affect the differentiation of mouse embryonic stem cells (ESCs) into HSCs. We prepared HSC-EXscon, HSC-EXssc and HSC-EXsmiR126 from control HSCs and the HSCs transfected with scramble control or miR126 mimics, respectively. HSC-EXs were isolated by ultracentrifugation and analyzed using nanoparticle tracking analysis. We incubated the collected EXs with mouse ESCs over a 10-d differentiation induction period, during which HSC-EXs and a Notch pathway activator (Jagged1, 100 ng/mL) were added to the cultures every 3 d. After the 10-d differentiation period, the expression levels of miR126, SSEA1, CD117, Sca1, Notch1 and Hes1 in ESCs were assessed. The generated HSCs were validated by flow cytometry using antibodies against HSC markers (CD117, CD34 and Sca1). Our results revealed that: (1) transfection with miR126 mimics significantly increased miR126 levels in HSC-EXsmiR126. (2) HSC-EX co-culture promoted mouse ESCs differentiation into HSCs with the most prominent effect found in the HSC-EXsmiR126 co-culture. (3) HSC differentiation was verified by reduced SSEA1 expression and increased CD117 and Sca1 expression. (4) All the effects caused by HSC-EXs were accompanied by significant reduction of Notch1 and Hes1 expression, thus inhibition of the Notch1/Hes1 pathway, whereas activation of Notch by Jagged1 abolished the effects of HSC-EXsmiR126. In conclusion, HSC-EXs promote hematopoietic differentiation of mouse ESCs in vitro by inhibiting the miR126/Notch1 pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest 2015; 125: 1243–54.
McKinney-Freeman S, Daley G . Derivation of hematopoietic stem cells from murine embryonic stem cells. J Vis Exp 2007; (2): 162.
Katsuda T, Kosaka N, Takeshita F, Ochiya T . The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013; 13: 1637–53.
Wang J, Chen S, Ma X, Cheng C, Xiao X, Chen J, et al. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev 2013; 2013: 572729.
Emanueli C, Shearn AI, Angelini GD, Sahoo S . Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol 2015; 71: 24–30.
Mause SF, Weber C . Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107: 1047–57.
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 2013; 31: 2737–46.
Wu K, Yang Y, Zhong Y, Ammar HM, Zhang P, Guo R, et al. The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. Am J Physiol Endocrinol Metab 2016; 310: E828–37.
Takeda YS, Xu Q . Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One 2015; 10: e0135111.
Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013; 121: 984–95.
Huang X, Gschweng E, Van HB, Cheng D, Mikkola HK, Witte ON . Regulated expression of microRNAs-126/126* inhibits erythropoiesis from human embryonic stem cells. Blood 2011; 117: 2157–65.
Chen CZ, Li L, Lodish HF, Bartel DP . MicroRNAs modulate hematopoietic lineage differentiation. Science 2004; 303: 83–6.
Nikolic I, Plate KH, Schmidt MH . EGFL7 meets miRNA-126: an angiogenesis alliance. J Angiogenes Res 2010; 2: 9.
Lechman ER, Gentner B, van Galen P, Giustacchini A, Saini M, Boccalatte FE, et al. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell 2012; 11: 799–811.
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med 2013; 31: 484–92.
Dikic I, Schmidt MH . Notch: Implications of endogenous inhibitors for therapy. Bioessays 2010; 32: 481–7.
Bray SJ . Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7: 678–89.
Ramasamy SK, Lenka N . Notch exhibits ligand bias and maneuvers stage-specific steering of neural differentiation in embryonic stem cells. Mol Cell Biol 2010; 30: 1946–57.
Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 1999; 11: 299–308.
Li H, Yu B, Zhang Y, Pan Z, Xu W, Li H . Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun 2006; 341: 320–5.
Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT . Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 2002; 99: 2369–78.
Ohishi K, Varnum-Finney B, Bernstein ID . Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38− cord blood cells. J Clin Invest 2002; 110: 1165–74.
Han W, Ye Q, Moore MA . A soluble form of human Delta-like-1 inhibits differentiation of hematopoietic progenitor cells. Blood 2000; 95: 1616–25.
Murry CE, Keller G . Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008; 132: 661–80.
Wang J, Guo R, Yang Y, Jacobs B, Chen S, Iwuchukwu I, et al. The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells Int 2016; 2016: 2639728.
Challen GA, Boles N, Lin KK, Goodell MA . Mouse hematopoietic stem cell identification and analysis. Cytometry A 2009; 75: 14–24.
Wang J, Zhong Y, Ma X, Xiao X, Cheng C, Chen Y, et al. Analyses of endothelial cells and endothelial progenitor cells released microvesicles by using microbead and Q-dot based nanoparticle tracking analysis. Sci Rep 2016; 6: 24679.
Vodyanik MA, Thomson JA, Slukvin II . Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006; 108: 2095–105.
Pan Q, Liu H, Zheng C, Zhao Y, Liao X, Wang Y, et al. Microvesicles derived from inflammation-challenged endothelial cells modulate vascular smooth muscle cell functions. Front Physiol 2016; 7: 692.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10: 619–24.
Lowell S, Benchoua A, Heavey B, Smith AG . Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol 2006; 4: e121.
Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, et al. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci U S A 2003; 100: 4018–23.
Nemir M, Croquelois A, Pedrazzini T, Radtke F . Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res 2006; 98: 1471–8.
Huang C, Jackson M, Samuel K, Taylor AH, Lowell S, Forrester LM . Haematopoietic differentiation is inhibited when Notch activity is enhanced in FLK1+ mesoderm progenitors. Stem Cell Res 2013; 11: 1273–87.
Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A . Signalling downstream of activated mammalian Notch. Nature 1995; 377: 355–8.
Kobayashi T, Mizuno H, Imayoshi I, Furusawa C, Shirahige K, Kageyama R . The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes Dev 2009; 23: 1870–5.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No 81400360); the Science and Technology Planning Project of Zhanjiang (No [2012] 172); and the Competitive Project of Zhanjiang (2014A01022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, Fl., Tan, L., Liu, H. et al. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharmacol Sin 39, 552–560 (2018). https://doi.org/10.1038/aps.2017.130
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.130
Keywords
This article is cited by
-
Characterization of the microRNA transcriptomes and proteomics of cochlear tissue-derived small extracellular vesicles from mice of different ages after birth
Cellular and Molecular Life Sciences (2022)
-
Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming
Tissue Engineering and Regenerative Medicine (2022)
-
Hematopoietic stem and progenitor cell signaling in the niche
Leukemia (2020)
-
Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis
Neuropsychopharmacology (2020)
-
Stem cell therapy: old challenges and new solutions
Molecular Biology Reports (2020)