Abstract
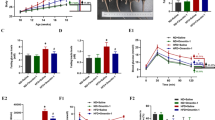
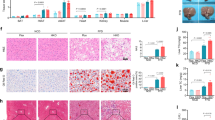
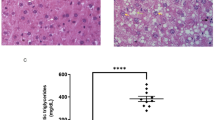
3-Acetyl-oleanolic acid (3Ac-OA) is a derivative of oleanolic acid (OA), which has shown therapeutic beneficial effects on diabetes and metabolic syndrome. In this study we investigated whether 3Ac-OA exerted beneficial effect on non-alcoholic fatty liver disease (NAFLD) in rats and its potential underlying mechanisms. Treatment with 3Ac-OA (1–100 μmol/L) dose-dependently decreased the intracellular levels of total cholesterol (TC) and triglyceride (TG) in FFA-treated primary rat hepatocytes and human HepG2 cell lines in vitro. Furthermore, oil red staining studies showed that 3Ac-OA caused dose-dependent decrease in the number of lipid droplets in FFA-treated primary rat hepatocytes. SD rats were fed a high fat diet (HFD) for 6 weeks and subsequently treated with 3Ac-OA (60, 30, 15 mg·kg-1·d-1) for 4 weeks. 3Ac-OA administration significantly decreased the body weight, liver weight and serum TC, TG, LDL-C levels in HFD rats. Furthermore, 3AcOA administration ameliorated lipid accumulation and cell apoptosis in the liver of HFD rats. Using adipokine array analyses, we found that the levels of 11 adipokines (HGF, ICAM, IGF-1, IGFBP-3, IGFBP-5, IGFBP-6, lipocalin-2, MCP-1, M-CSF, Pref-1 and RAGE) were increased by more than twofold in the serum of 3Ac-OA-treated rats, whereas ICAM, IGF-1 and lipocalin-2 had levels increased by more than 20-fold. Moreover, 3Ac-OA administration significantly increased the expression of glucose transporter type 2 (GLUT-2) and low-density lipoprotein receptor (LDLR), as well as the phosphorylation of AMP-activated protein kinase (AMPK), protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3β) in the liver tissues of HFD rats. In conclusion, this study demonstrates that 3Ac-OA exerts a protective effect against hyperlipidemia in NAFLD rats through AMPK-related pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21: 3777–85.
Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006; 131: 934–45.
Barritt ASt, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol 2011; 45: 372–8.
Piccinin E, Moschetta A. Hepatic-specific PPARalpha-FGF21 action in NAFLD. Gut 2016; 65: 1075–6.
Miyake T, Kumagi T, Hirooka M, Furukawa S, Yoshida O, Koizumi M, et al. Low alcohol consumption increases the risk of impaired glucose tolerance in patients with non-alcoholic fatty liver disease. J Gastroenterol 2016; 51: 1090–100.
Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016; 61: 1294–303.
Chen S, Wen X, Zhang W, Wang C, Liu J, Liu C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1beta axis in high-fat diet-induced hyperlipidemic mice. FASEB J 2016; 31: 1085–96.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–51.
Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res 2014; 58: 1750–9.
Jiang Q, Wang D, Han Y, Han Z, Zhong W, Wang C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int J Biochem Cell Biol 2015; 69: 142–52.
Zhang L, Dong J, Zhang Y, Liu J, Sun H. Design, synthesis, and biological evaluation of potent photoaffinity probes of oleanolic acid. Med Chem 2013; 9: 294–302.
Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res 2015; 2015: 973287.
Cheng K, Liu J, Liu X, Li H, Sun H, Xie J. Synthesis of glucoconjugates of oleanolic acid as inhibitors of glycogen phosphorylase. Carbohydr Res 2009; 344: 841–50.
Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V. Loss of estrogen-related receptor alpha promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci U S A 2013; 110: 17975–80.
Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, et al. Prevention of free fatty acid–induced hepatic lipotoxicity by 18β-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 2008; 47: 1905–15.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015, 313: 2263–73
Ferslew BC, Johnston CK, Tsakalozou E, Bridges AS, Paine MF, Jia W, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther 2015; 97: 419–27.
Lukacs-Kornek V, Schuppan D. Dendritic cells in liver injury and fibrosis: shortcomings and promises. J Hepatol 2013; 59: 1124–6.
Tarantino G, Citro V, Finelli C. Recreational drugs: a new health hazard for patients with concomitant chronic liver diseases. J Gastrointestin Liver Dis 2014; 23: 79–84.
Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr 2011; 53: 131–40.
Wang JW, Wan XY, Zhu HT, Lu C, Yu WL, Yu CH, et al. Lipotoxic effect of p21 on free fatty acid-induced steatosis in L02 cells. PLoS One 2014; 9: e96124.
Chu JH, Wang H, Ye Y, Chan PK, Pan SY, Fong WF, et al. Inhibitory effect of schisandrin B on free fatty acid-induced steatosis in L-02 cells. World J Gastroenterol 2011; 17: 2379–88.
Romaniuk D, Kimsa MW, Strzalka-Mrozik B, Kimsa MC, Kabiesz A, Romaniuk W, et al. Gene expression of IGF1, IGF1R, and IGFBP3 in epiretinal membranes of patients with proliferative diabetic retinopathy: preliminary study. Mediators Inflamm 2013; 2013: 986217.
Jeon SM, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res 2015; 38: 346–57.
King TS, Russe OQ, Moser CV, Ferreiros N, Kynast KL, Knothe C, et al. AMP-activated protein kinase is activated by non-steroidal anti-inflammatory drugs. Eur J Pharmacol 2015; 762: 299–305.
Basta HA, Palmenberg AC. AMP-activated protein kinase phosphorylates EMCV, TMEV and SafV leader proteins at different sites. Virology 2014; 462-463: 236–40.
Landgraf RR, Goswami D, Rajamohan F, Harris MS, Calabrese MF, Hoth LR, et al. Activation of AMP-activated protein kinase revealed by hydrogen/deuterium exchange mass spectrometry. Structure 2013; 21: 1942–53.
Brabant G, Muller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. FASEB J 2005; 19: 1048–50.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81673443). We also thank Dr Hong-bin SUN for providing compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ou-Yang, Q., Xuan, Cx., Wang, X. et al. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin 39, 1284–1293 (2018). https://doi.org/10.1038/aps.2017.142
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.142
Keywords
This article is cited by
-
Oleanolic acid improved intestinal immune function by activating and potentiating bile acids receptor signaling in E. coli-challenged piglets
Journal of Animal Science and Biotechnology (2024)
-
Therapeutic potential of oleanolic acid in liver diseases
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Metformin induces pyroptosis in leptin receptor-defective hepatocytes via overactivation of the AMPK axis
Cell Death & Disease (2023)
-
Linalool Mitigated High-Fat Diet–Induced Non-alcoholic Fatty Liver Disease by Regulating the Intestinal-Hepatic Axis via TGF-β/NF-kB/TLR4/ZO-1 Pathway
Revista Brasileira de Farmacognosia (2023)