Abstract
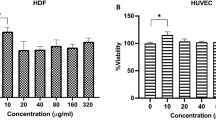
Wound therapy remains a clinical challenge due to the complexity of healing pathology and high demand of achieving functional and aesthetically satisfactory scars. Newly formed blood vessels are essential for tissue repair since they can support cells at the wound site with nutrition and oxygen. In this study, we investigated the effects of Asperosaponin VI (ASA VI) isolated from a traditional Chinese medicine, the root of Dipsacus asper Wall, in promoting angiogenesis, as well as its function in wound therapeutics. Treatment of human umbilical vein endothelial cells (HUVECs) with ASA VI (20–80 μg/mL) dose-dependently promoted the proliferation, migration and enhanced their angiogenic ability in vitro, which were associated with the up-regulated HIF-1α/VEGF signaling. Full-thickness cutaneous wound model rats were injected with ASA VI (20 mg·kg−1·d−1, iv) for 21 d. Administration of ASA VI significantly promoted the cutaneous wound healing, and more blood vessels were observed in the regenerated tissue. Due to rapid vascularization, the cellular proliferation status, granulation tissue formation, collagen matrix deposition and remodeling processes were all accelerated, resulting in efficient wound healing. In summary, ASA VI promotes angiogenesis of HUVECs in vitro via up-regulating the HIF-1α/VEGF pathway, and efficiently enhances the vascularization in regenerated tissue and facilitates wound healing in vivo. The results reveal that ASA VI is a potential therapeutic for vessel injury-related wounds.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
15 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41401-021-00777-3
References
Eberhardt RT, Raffetto JD . Chronic venous insufficiency. Circulation 2014; 130: 333–46.
Valencia IC, Falabella A, Kirsner RS, Eaglstein WH . Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001; 44: 401–24.
Muralidhar A, Babu KS, Sankar TR, Reddanna P, Latha J . Evaluation of wound healing properties of bioactive fractions from the extract of Butea monosperma (lam) stem bark. Int J Phytomed 2011; 3: 41.
Hwang SH, Lee BH, Choi SH, Kim HJ, Won KJ, Lee HM, et al. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res 2016; 40: 325–33.
Hosemann W, Wigand M, Göde U, Linger F, Dunker I . Normal wound healing of the paranasal sinuses: clinical and experimental investigations. Eur Arch Otorhinolaryngol 1991; 248: 390–4.
Watelet JB, Bachert C, Gevaert P, Van Cauwenberge P . Wound healing of the nasal and paranasal mucosa: a review. Am J Rhinol 2002; 16: 77–84.
Kim BS, Pallua N, Bernhagen J, Bucala R . The macrophage migration inhibitory factor protein superfamily in obesity and wound repair. Exp Mol Med 2015; 47: e161.
Bates DO, Jones RO . The role of vascular endothelial growth factor in wound healing. Int J Low Extrem Wounds 2003; 2: 107–20.
Hwang J, Kim CW, Son KN, Han KY, Lee KH, Kleinman HK, et al. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett 2004; 570: 47–51.
Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states. Circulation 2002; 105: 373–9.
Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC . Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 2003; 162: 303–12.
Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marcé M, Semenza GL . Adenoviral transfer of HIF-1α enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A 2009; 106: 18769–74.
Rey S, Semenza GL . Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodeling. Cardiovasc Res 2010; 86: 236–42.
Shweiki D, Itin A, Soffer D, Keshet E . Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992; 359: 843.
Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care 2014; 3: 390–9.
Bir SC, Pattillo CB, Pardue S, Kolluru GK, Shen X, Giordano T, et al. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes 2014; 63: 270–81.
Yang HN, Park JS, Woo DG, Jeon SY, Park KH . Transfection of VEGF 165 genes into endothelial progenitor cells and in vivo imaging using quantum dots in an ischemia hind limb model. Biomaterials 2012; 33: 8670–84.
Garbern JC, Minami E, Stayton PS, Murry CE . Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 2011; 32: 2407–16.
Won YW, McGinn AN, Lee M, Nam K, Bull DA, Kim SW . Post-translational regulation of a hypoxia-responsive VEGF plasmid for the treatment of myocardial ischemia. Biomaterials 2013; 34: 6229–38.
Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 2007; 120: 964–72.
Epstein SE, Kornowski R, Fuchs S, Dvorak HF . Angiogenesis therapy. Circulation 2001; 104: 115–9.
Kim YS, Cho IH, Jeong MJ, Jeong SJ, Nah SY, Cho YS, et al. Therapeutic effect of total ginseng saponin on skin wound healing. J Ginseng Res 2011; 35: 360–7.
Xing SS, Yang XY, Zheng T, Li WJ, Wu D, Chi JY, et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vasc Pharmacol 2015; 72: 141–52.
Namba T . The Encyclopedia of Wakan-Yaku: traditional Sino-Japanese medicines, with color pictures, Vol I. Hoikusha Publishing Company, Osaka, Japan. 1993; 185–6.
Jung KY, Do JC, Son KH . Triterpene glycosides from the roots of Dipsacus asper. J Nat Prod 1993; 56: 1912–6.
Suh HW, Song DK, Huh SO, Son KH, Kim YH . Antinociceptive mechanisms of Dipsacus saponin C administered intrathecally in mice. J Ethnopharmacol 2000; 71: 211–8.
Yu X, Wang LN, Ma L, You R, Cui R, Ji D, et al. Akebia saponin D attenuates ibotenic acid-induced cognitive deficits and pro-apoptotic response in rats: involvement of MAPK signal pathway. Pharmacol Biochem Behav 2012; 101: 479–86.
Li C, Tian J, Li G, Jiang W, Xing Y, Hou J, et al. Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways. Eur J Pharmacol 2010; 649: 100–7.
Tang Y, Huang B, Sun L, Peng X, Chen X, Zou X . Ginkgolide B promotes proliferation and functional activities of bone marrow-derived endothelial progenitor cells: involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell Mater 2011; 21: 459–69.
Li C, Liu ZJ, Li G, Jiang W, Zhang G, Chen F, et al. Protective roles of Asperosaponin VI, a triterpene saponin isolated from Dipsacus asper Wall on acute myocardial infarction in rats. Eur J Pharmacol 2010; 627: 235–41.
Gurtner GC, Werner S, Barrandon Y, Longaker MT . Wound repair and regeneration. Nature 2008; 453: 314–21.
Zeng Z, Zhu BH . Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. J Ethnopharmacol 2014; 154: 653–62.
Morimoto N, Yoshimura K, Niimi M, Ito T, Tada H, Teramukai S, et al. An exploratory clinical trial for combination wound therapy with a novel medical matrix and fibroblast growth factor in patients with chronic skin ulcers: a study protocol. Am J Transl Res 2012; 4: 52.
Lamalice L, Le Boeuf F, Huot J . Endothelial cell migration during angiogenesis. Circ Res 2007; 100: 782–94.
Ingber DE . Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 2002; 91: 877–87.
Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 2008; 105: 19426–31.
Chen H, Jia P, Kang H, Zhang H, Liu Y, Yang P, et al. Upregulating Hif-1α by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016; 5: 907–18.
Leivonen SK, Häkkinen L, Liu D, Kähäri VM . Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-β-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol 2005; 124: 1162–9.
Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol 2009; 129: 2275–87.
Werner S, Krieg T, Smola H . Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 2007; 127: 998–1008.
Clark RA . Basics of cutaneous wound repair. J Dermatol Surg Oncol 1993; 19: 693–706.
Merkel JR, DiPaolo BR, Hallock GG, Rice DC . Type I and Type III collagen content of healing wounds in fetal and adult rats 1. Proc Soc Exp Biol Med 1988; 187: 493–7.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81572227), the Zhejiang Provincial Natural Science Foundation (LY14H170002), the Zhejiang Undergraduate Talent Project (2017R413071), and the Wenzhou Science and Technology Bureau Project (Y20160063).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Cg., Lou, Yt., Tong, Mj. et al. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1α/VEGF signaling. Acta Pharmacol Sin 39, 393–404 (2018). https://doi.org/10.1038/aps.2017.161
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.161
Keywords
This article is cited by
-
Asperosaponin VI mitigates mitochondrial dysfunction and chondrocyte apoptosis in osteoarthritis by modulating the AMPK-SIRT3 pathway
Cell Biology and Toxicology (2025)
-
Mechanism of Drilling Parameter Distortion Induced by Drill Rod Buckling While MWD Application
Rock Mechanics and Rock Engineering (2025)
-
A critical overview of challenging roles of medicinal plants in improvement of wound healing technology
DARU Journal of Pharmaceutical Sciences (2024)
-
Intramuscular injection of sotagliflozin promotes neovascularization in diabetic mice through enhancing skeletal muscle cells paracrine function
Acta Pharmacologica Sinica (2022)
-
In the presence of TGF-β1, Asperosaponin VI promotes human mesenchymal stem cell differentiation into nucleus pulposus like- cells
BMC Complementary Medicine and Therapies (2021)