Abstract
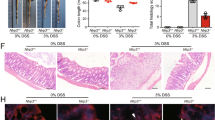
Ulcerative colitis (UC) is a chronic, nonspecific inflammatory bowel disease (IBD) characterized by complicated and relapsing inflammation in the gastrointestinal tract. SM934 is a water-soluble artemisinin analogue that shows anti-inflammatory and immuno-regulatory effects. In this study, we investigated the effects of SM934 on UC both in vivo and in vitro. A mouse model of colitis was established in mice by oral administration of 5% dextran sulfate sodium (DSS). SM934 (3, 10 mg/kg per day, ig) was administered to the mice for 10 days. After the mice were sacrificed, colons, spleens and mesenteric lymph nodes (MLNs) were collected for analyses. We showed that SM934 administration restored DSS-induced body weight loss, colon shortening, injury and inflammation scores. Furthermore, SM934 administration significantly decreased the disease activity index (DAI), histopathological scores, and myeloperoxidase (MPO) activities in colonic tissues. Moreover, SM934 administration dose-dependently decreased the mRNA and protein levels of DSS-induced pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), and the percentage of macrophages and neutrophils in colon tissues. The effects of SM934 on LPS-stimulated RAW 264.7 cells and THP-1-derived macrophages were examined in vitro. Treatment with SM934 (0.8, 8, 80 μmol/L) dose-dependently decreased the production of pro-inflammatory mediators in LPS-stimulated RAW264.7 cells and THP-1-derived macrophages via inhibiting activation of the NF-κB signaling. Our results reveal the protective effects of SM934 on DSS-induced colitis can be attributed to its suppressing effects on neutrophils and macrophages and its inhibitory role in the NF-κB signaling, suggests that SM934 might be a potential effective drug for ulcerative colitis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–29.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007; 448: 427–34.
Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 2007; 59: 1073–83.
Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol 1995; 10: 387–95.
Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14: 329–42.
Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis 2000; 6: 21–33.
Schottelius AJ, Dinter H. Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res 2006; 130: 67–87.
Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998; 115: 357–69.
Neurath MF, Fuss I, Schurmann G, Pettersson S, Arnold K, Muller-Lobeck H, et al. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci 1998; 859: 149–59.
Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med 1996; 2: 998–1004.
Li TT, Zhang XH, Jing JF, Li X, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates the proteinuria and renal fibrosis in rat experimental membranous nephropathy. Acta Pharmacol Sin 2015; 36: 188–99.
Li X, Li TT, Zhang XH, Hou LF, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS One 2013; 8: e74108.
Lin ZM, Yang XQ, Zhu FH, He SJ, Tang W, Zuo JP. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci Rep 2016; 6: 38115.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–55.
Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z, et al. Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation. Cell Mol Immunol 2016; 13: 379–90.
Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol 1999; 127: 1915–21.
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014; 104: Unit 15 25.
Zhang L, Zhang Y, Zhong W, Di C, Lin X, Xia Z. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem 2014; 289: 26847–58.
Kane S, Marchioni Beery R. Current approaches to the management of new-onset ulcerative colitis. Clin Exp Gastroenterol 2014; 7: 111–32.
Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, et al. Galectin-1 suppresses experimental colitis in mice. Gastroenterology 2003; 124: 1381–94.
Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 2012; (60). pii: 3678. doi: 10.3791/3678.
Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol 2001; Chapter 3: Unit 3.19.
Qi Q, Li H, Lin ZM, Yang XQ, Zhu FH, Liu YT, et al. (5R)-5-hydroxytriptolide ameliorates anti-glomerular basement membrane glomerulonephritis in NZW mice by regulating Fcgamma receptor signaling. Acta Pharmacol Sin 2018; 39: 107–16.
Wang JX, Hou LF, Yang Y, Tang W, Li Y, Zuo JP. SM905, an artemisinin derivative, inhibited NO and pro-inflammatory cytokine production by suppressing MAPK and NF-kappaB pathways in RAW 264.7 macrophages. Acta Pharmacol Sin 2009; 30: 1428–35.
Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 2009; 4: e6073.
Serra AM, Waddell J, Manivannan A, Xu H, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol 2012; 181: 719–27.
Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med 2008; 263: 591–6.
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010; 59: 1192–9.
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015; 12: 205–17.
Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994; 107: 1643–52.
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 2014; 13: 3–10.
Li H, Zuo J, Tang W. Water-soluble artemisinin derivatives as promising therapeutic immunosuppressants of autoimmune diseases. Cell Mol Immunol 2017 Sep 11. doi: 10.1038/cmi.2017.87.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–55.
Wei W, Ding M, Zhou K, Xie H, Zhang M, Zhang C. Protective effects of wedelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling. Biomed Pharmacother 2017; 94: 27–36.
Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010; 10: 735–44.
Ajuebor MN, Virag L, Flower RJ, Perretti M, Szabo C. Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 1998; 95: 625–30.
Zhang DK, Cheng LN, Huang XL, Shi W, Xiang JY, Gan HT. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Int J Colorectal Dis 2009; 24: 5–12.
Acknowledgements
This work was supported by grants “Personalized Medicines-Molecular Signature-based Drug Discovery and Development”, Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No XDA12020107, National Science & Technology Major Project “New Drug Creation and Manufacturing Program”, China (No 2017ZX09101002-002-010), National Natural Science Foundation of China (NSFC): 81673445 and we are grateful to Dr Lin WANG (University of Michigan) who kindly contributed to the manuscript revision.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, Yx., Shao, Mj., Qi, Q. et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin 39, 1633–1644 (2018). https://doi.org/10.1038/aps.2017.185
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.185
Keywords
This article is cited by
-
Rebalancing the inflammatory trajectory from inflammatory bowel disease to colitis-associated colorectal cancer via artemisinin-based multitarget therapy
Acta Pharmacologica Sinica (2026)
-
Ardisiacrispin B, a natural triterpenoid saponins, suppresses dextran sulfate sodium-induced inflammatory bowel disease by rebalancing the gut microbiota and Th17/Treg of mice
Inflammopharmacology (2026)
-
TLR4/NF-κB-mediated M1 macrophage polarization contributes to the promotive effects of ETS2 on ulcerative colitis
European Journal of Medical Research (2025)
-
Lactobacillus acidophilus extracellular vesicles-coated UiO-66-NH2@siRNA nanoparticles for ulcerative colitis targeted gene therapy and gut microbiota modulation
Journal of Nanobiotechnology (2025)
-
Immunotherapy for ulcerative colitis: targeting innate and adaptive immune responses for better clinical outcomes
The Egyptian Journal of Internal Medicine (2025)