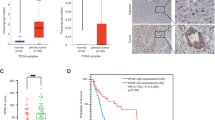
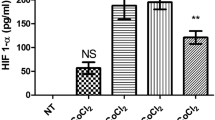
Abstract
Aggregated metastatic cancer cells, referred to as circulating tumor cell (CTC) clusters, are present in the blood of cancer patients and contribute to cancer metastasis. However, the origin of CTC clusters, especially intravascular aggregates, remains unknown. Here, we employ suspension culture methods to mimic CTC cluster formation in the circulation of breast cancer patients. CTC clusters generated using these methods exhibited an increased metastatic potential that was defined by the overexpression of heparanase (HPSE). Heparanase induced FAK- and ICAM-1-dependent cell adhesion, which promoted intravascular cell aggregation. Moreover, knockdown of heparanase or inhibition of its activity with JG6, a heparanase inhibitor, was sufficient to block the formation of cell clusters and suppress breast cancer metastasis. Our data reveal that heparanase-mediated cell adhesion is critical for metastasis mediated by intravascular CTC clusters. We also suggest that targeting the function of heparanase in cancer cell dissemination might limit metastatic progression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74.
Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016; 529: 298–306.
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014; 158: 1110–22.
Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci U S A 2012; 109: E2595–604.
Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH, Yun SH. Urokinase exerts antimetastatic effects by dissociating clusters of circulating tumor cells. Cancer Res 2015; 75: 4474–82.
Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A 2002; 99: 2193–8.
Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer 1973; 11: 704–18.
Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011; 178: 989–96.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013; 339: 580–4.
Fukunaga Y, Liu H, Shimizu M, Komiya S, Kawasuji M, Nagafuchi A. Defining the roles of beta-catenin and plakoglobin in cell-cell adhesion: isolation of beta-catenin/plakoglobin-deficient F9 cells. Cell Struct Funct 2005; 30: 25–34.
Lu L, Zeng H, Gu X, Ma W. Circulating tumor cell clusters-associated gene plakoglobin and breast cancer survival. Breast Cancer Res Treat 2015; 151: 491–500.
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 2016; 113: E854–63.
Vornicova O, Boyango I, Feld S, Naroditsky I, Kazarin O, Zohar Y, et al. The prognostic significance of heparanase expression in metastatic melanoma. Oncotarget 2016; 7: 74678–85.
Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell B 2006; 38: 2018–39.
Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, et al. Heparanase mediates cell adhesion independent of its enzymatic activity. FASEB J 2003; 17: 1015–25.
Levy-Adam F, Feld S, Suss-Toby E, Vlodavsky I, Ilan N. Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PLoS One 2008; 3: e2319.
Zetser A, Bashenko Y, Miao HQ, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res 2003; 63: 7733–41.
Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18: 1217–23.
Mu Z, Wang C, Ye Z, Austin L, Civan J, Hyslop T, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat 2015; 154: 563–71.
Magbanua MJM, Carey LA, DeLuca A, Hwang J, Scott JH, Rimawi MF, et al. Circulating tumor cell analysis in metastatic triple-negative breast cancers. Clin Cancer Res 2015; 21: 1098–105.
Sun X, Zhang G, Nian J, Yu M, Chen S, Zhang Y, et al. Elevated heparanase expression is associated with poor prognosis in breast cancer: a study based on systematic review and TCGA data. Oncotarget 2017; 8: 43521–35.
Levy-Adam F, Ilan N, Vlodavsky I. Tumorigenic and adhesive properties of heparanase. Semin Cancer Biol 2010; 20: 153–60.
Li QN, Liu HY, Xin XL, Pan QM, Wang L, Zhang J, et al. Marine-derived oligosaccharide sulfate (JG3) suppresses heparanase-driven cell adhesion events in heparanase over-expressing CHO-K1 cells. Acta Pharmacol Sin 2009; 30: 1033–8.
Reiland J, Kempf D, Roy M, Denkins Y, Marchetti D. FGF2 binding, signaling, and angiogenesis are modulated by heparanase in metastatic melanoma cells. Neoplasia 2006; 8: 596–606.
Zhao HJ, Liu HY, Chen Y, Xin XL, Li J, Hou YT, et al. Oligomannurarate sulfate, a novel heparanase inhibitor simultaneously targeting basic fibroblast growth factor, combats tumor angiogenesis and metastasis. Cancer Res 2006; 66: 8779–87.
Khamaysi I, Singh P, Nasser S, Awad H, Chowers Y, Sabo E, et al. The role of heparanase in the pathogenesis of acute pancreatitis: a potential therapeutic target. Sci Rep-Uk 2017; 7: 715.
Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J 2017; 284: 42–55.
Liu CJ, Chang J, Lee PH, Lin DY, Wu CC, Jeng LB, et al. Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J Gastroentero 2014; 20: 11384–93.
Lewis KD, Robinson WA, Millward MJ, Powell A, Price TJ, Thomson DB, et al. A phase II study of the heparanase inhibitor PI-88 in patients with advanced melanoma. Invest New Drug 2008; 26: 89–94.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012; 30: 525–32.
Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016; 16: 433.
Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat 2017; 161: 83–94.
Fabisiewicz A, Grzybowska E. CTC clusters in cancer progression and metastasis. Med Oncol 2017; 34: 12.
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005; 6: 56–68.
Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011; 63: 610–5.
Luo M, Guan JL. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 2010; 289: 127–39.
Jia L, Ma S. Recent advances in the discovery of heparanase inhibitors as anti-cancer agents. Eur J Med Chem 2016; 121: 209–20.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81302791), the Youth Innovation Promotion Association CAS, the Strategic Priority Research Program of the Chinese Academy of Sciences (No XDA12020326) and the Shanghai Talent Development Funds (No 201663), all awarded to Xun HUANG.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Supplementary Figures
Supplementary Figures S1–S4
Rights and permissions
About this article
Cite this article
Wei, Rr., Sun, Dn., Yang, H. et al. CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacol Sin 39, 1326–1337 (2018). https://doi.org/10.1038/aps.2017.189
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.189
Keywords
This article is cited by
-
PAK2 promotes CTC cluster formation by phosphorylating E-cadherin to enhance cell-cell adhesion in breast cancer
Breast Cancer Research (2025)
-
Clinical applications of circulating tumor cells in metastasis and therapy
Journal of Hematology & Oncology (2025)
-
Mechanisms of breast cancer metastasis: the role of extracellular matrix
Molecular and Cellular Biochemistry (2025)
-
Heparanase2 inhibits the growth of gallbladder cancer by regulating the VEGF/VEGFR pathway
Discover Oncology (2025)
-
Systematic optimization and evaluation of culture conditions for the construction of circulating tumor cell clusters using breast cancer cell lines
BMC Cancer (2024)