Abstract
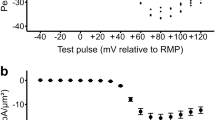
Emerging evidence suggests that Ca2+ signals are important for the self-renewal and differentiation of human embryonic stem cells (hESCs). However, little is known about the physiological and pharmacological properties of the Ca2+-handling machinery in hESCs. In this study we used RT-PCR and Western blotting to analyze the expression profiles of genes encoding Ca2+-handling proteins; we also used confocal Ca2+ imaging and pharmacological approaches to determine the contribution of the Ca2+-handling machinery to the regulation of Ca2+ signaling in hESCs. We revealed that hESCs expressed pluripotent markers and various Ca2+-handling-related genes. ATP-induced Ca2+ transients in almost all hESCs were inhibited by the inositol-1,4,5-triphosphate receptor (IP3R) blocker 2-APB or xestospongin C. In addition, Ca2+ transients were induced by a ryanodine receptor (RyR) activator, caffeine, in 10%–15% of hESCs and were blocked by ryanodine, whereas caffeine and ATP did not have additive effects. Moreover, store-operated Ca2+ entry (SOCE) but not voltage-operated Ca2+ channel-mediated Ca2+ entry was observed. Inhibition of sarco/endoplasmic reticulum (ER) Ca2+-ATPase (SERCA) by thapsigargin induced a significant increase in the cytosolic free Ca2+ concentration ([Ca2+]i). For the Ca2+ extrusion pathway, inhibition of plasma membrane Ca2+ pumps (PMCAs) by carboxyeosin induced a slow increase in [Ca2+]i, whereas the Na+/Ca2+ exchanger (NCX) inhibitor KBR7943 induced a rapid increase in [Ca2+]i. Taken together, increased [Ca2+]i is mainly mediated by Ca2+ release from intracellular stores via IP3Rs. In addition, RyRs function in a portion of hESCs, thus indicating heterogeneity of the Ca2+-signaling machinery in hESCs; maintenance of low [Ca2+]i is mediated by uptake of cytosolic Ca2+ into the ER via SERCA and extrusion of Ca2+ out of cells via NCX and PMCA in hESCs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bellamy V, Vanneaux V, Bel A, Nemetalla H, Emmanuelle Boitard S, Farouz Y, et al. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Heart Lung Transplant 2015; 34: 1198–207.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015; 36: 2011–7.
Passier R, van Laake LW, Mummery CL . Stem-cell-based therapy and lessons from the heart. Nature 2008; 453: 322–9.
Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest 2010; 120: 1125–39.
Huang J, Zhang M, Zhang P, Liang H, Ouyang K, Yang HT . Coupling switch of P2Y-IP3 receptors mediates differential Ca2+ signaling in human embryonic stem cells and derived cardiovascular progenitor cells. Purinergic Signal 2016; 12: 465–78.
Apati A, Berecz T, Sarkadi B . Calcium signaling in human pluripotent stem cells. Cell Calcium 2016; 59: 117–23.
Apati A, Paszty K, Hegedus L, Kolacsek O, Orban TI, Erdei Z, et al. Characterization of calcium signals in human embryonic stem cells and in their differentiated offspring by a stably integrated calcium indicator protein. Cell Signal 2013; 25: 752–9.
Ermakov A, Pells S, Freile P, Ganeva VV, Wildenhain J, Bradley M, et al. A role for intracellular calcium downstream of G-protein signaling in undifferentiated human embryonic stem cell culture. Stem Cell Res 2012; 9: 171–84.
Apati A, Paszty K, Erdei Z, Szebenyi K, Homolya L, Sarkadi B . Calcium signaling in pluripotent stem cells. Mol Cell Endocrinol 2012; 353: 57–67.
Yanagida E, Shoji S, Hirayama Y, Yoshikawa F, Otsu K, Uematsu H, et al. Functional expression of Ca2+ signaling pathways in mouse embryonic stem cells. Cell Calcium 2004; 36: 135–46.
Tonelli FM, Santos AK, Gomes DA, da Silva SL, Gomes KN, Ladeira LO, et al. Stem cells and calcium signaling. Adv Exp Med Biol 2012; 740: 891–916.
Kapur N, Mignery GA, Banach K . Cell cycle-dependent calcium oscillations in mouse embryonic stem cells. Am J Physiol Cell Physiol 2007; 292: C1510–8.
Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, et al. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res 2013; 23: 1119–32.
Li K, Zhang W, Liu J, Wang W, Xie W, Fang H, et al. Flash sniper: automated detection and analysis of mitochondrial superoxide flash. Biophys J 2009; 96: 531a–32a.
Mikoshiba K . IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem 2007; 102: 1426–46.
Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 1997; 19: 723–33.
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL . Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2010; 2: a003996.
Rossier MF . T-type calcium channel: a privileged gate for calcium entry and control of adrenal steroidogenesis. Front Endocrinol 2016; 7: 43.
Dolphin AC . L-type calcium channel modulation. Adv Second Messenger Phosphoprotein Res 1999; 33: 153–77.
Ong HL, de Souza LB, Ambudkar IS . Role of TRPC channels in store-operated calcium entry. Adv Exp Med Biol 2016; 898: 87–109.
Sabourin J, Bartoli F, Antigny F, Gomez AM, Benitah JP . Transient receptor potential canonical (TRPC)/Orai1-dependent store-operated Ca2+ channels: new targets of aldosterone in cardiomyocytes. J Biol Chem 2016; 291: 13394–409.
Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, et al. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A 2002; 99: 9225–30.
Michelangeli F, East JM . A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans 2011; 39: 789–97.
Green AK, Cobbold PH, Dixon CJ . Effects on the hepatocyte [Ca2+]i oscillator of inhibition of the plasma membrane Ca2+ pump by carboxyeosin or glucagon-(19–29). Cell Calcium 1997; 22: 99–109.
Sibarov DA, Abushik PA, Poguzhelskaya EE, Bolshakov KV, Antonov SM . Inhibition of plasma membrane Na/Ca-exchanger by KB-R7943 or lithium reveals its role in Ca-dependent N-methyl-D-aspartate receptor Inactivation. J Pharmacol Exp Ther 2015; 355: 484–95.
Mamo S, Kobolak J, Borbiro I, Biro T, Bock I, Dinnyes A . Gene targeting and calcium handling efficiencies in mouse embryonic stem cell lines. World J Stem Cells 2010; 2: 127–40.
Rodriguez-Gomez JA, Levitsky KL, Lopez-Barneo J . T-type Ca2+ channels in mouse embryonic stem cells: modulation during cell cycle and contribution to self-renewal. Am J Physiol Cell Physiol 2012; 302: C494–504.
Forostyak O, Romanyuk N, Verkhratsky A, Sykova E, Dayanithi G . Plasticity of calcium signaling cascades in human embryonic stem cell-derived neural precursors. Stem Cells Dev 2013; 22: 1506–21.
Catterall WA, Swanson TM . Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol Pharmacol 2015; 88: 141–50.
Hogan PG, Rao A . Store-operated calcium entry: mechanisms and modulation. Biochem Biophys Res Commun 2015; 460: 40–9.
Albarran L, Lopez JJ, Salido GM, Rosado JA . Historical overview of store-operated Ca2+ entry. Adv Exp Med Biol 2016; 898: 3–24.
Lopez JJ, Albarran L, Gomez LJ, Smani T, Salido GM, Rosado JA . Molecular modulators of store-operated calcium entry. Biochim Biophys Acta 2016; 1863: 2037–43.
Foskett JK, White C, Cheung KH, Mak DO . Inositol trisphosphate receptor Ca2+ release channels. Phys Rev 2007; 87: 593–658.
Berridge MJ, Lipp P, Bootman MD . The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11–21.
Klishin A, Sedova M, Blatter LA . Time-dependent modulation of capacitative Ca2+ entry signals by plasma membrane Ca2+ pump in endothelium. Am J Physiol 1998; 274: C1117–28.
Brini M, Carafoli E . The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 2011; 3. Doi: 10.1101/cshperspect.a004168.
Acknowledgements
This work was supported by grants from the National Natural Science of China (No 81520108004, 81470422 and 31030050 to HTY, No 31401167 to MZ), the National Basic Research Program of China (No 2014CB965100 to Huang-tian YANG), the National Science and Technology Major Project (No 2012ZX09501001 to Huang-tian YANG), the National Key Research and Development Program (2016YFC1301200 to Huang-tian YANG), and the Natural Science Foundation of Shanghai (No 17ZR1435500 to Jin-jun HUANG).
We thank WiCell Research Institute for providing the H7 and H9 hESCs and Dr He-ping CHENG (Peking University, Beijing, China) for providing the Flash Sniper software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Jj., Wang, Yj., Zhang, M. et al. Functional expression of the Ca2+ signaling machinery in human embryonic stem cells. Acta Pharmacol Sin 38, 1663–1672 (2017). https://doi.org/10.1038/aps.2017.29
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.29
Keywords
This article is cited by
-
Myosin light chain 2 marks differentiating ventricular cardiomyocytes derived from human embryonic stem cells
Pflügers Archiv - European Journal of Physiology (2021)
-
TATA box-binding protein-related factor 3 drives the mesendoderm specification of human embryonic stem cells by globally interacting with the TATA box of key mesendodermal genes
Stem Cell Research & Therapy (2020)
-
Minimal contribution of IP3R2 in cardiac differentiation and derived ventricular-like myocytes from human embryonic stem cells
Acta Pharmacologica Sinica (2020)
-
Acoustic Tweezing Cytometry Induces Rapid Initiation of Human Embryonic Stem Cell Differentiation
Scientific Reports (2018)