Abstract
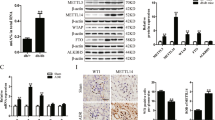
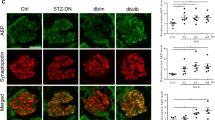
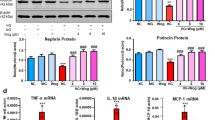
Glomerular epithelial podocytes are highly specialized cells that play a crucial role in maintaining normal function of the glomerular filtration barrier via their foot processes. Chrysin (5,7-dihydroxyflavone) is a natural flavonoid found in propolis and mushrooms that has anti-inflammatory, antioxidant and anticancer properties. This study aimed to evaluate the renoprotective effects of chrysin on podocyte apoptotic loss and slit diaphragm protein deficiency in high glucose-exposed podocytes and in db/db mouse kidneys. Exposure to high glucose (33 mmol/L) caused glomerular podocyte apoptosis in vitro, which was dose-dependently attenuated by nontoxic chrysin (1–20 μmol/L) through reduction of DNA fragmentation. Chrysin treatment dose-dependently restored the increased Bax/Bcl-2 ratio, and suppressed Apaf-1 induction and the elevated cytochrome c release in high glucose-exposed renal podocytes. In diabetic db/db mice, oral administration of chrysin (10 mg·kg−1·d−1, for 10 weeks) significantly attenuated proteinuria, and alleviated the abnormal alterations in glomerular ultrastructure, characterized by apoptotic podocytes and foot process effacement. In addition, this compound improved the induction of slit diaphragm proteins podocin/nephrin in the diabetic glomeruli. Exposure to high glucose elevated the unfolded protein response (UPR) to ER stress in renal podocytes, evidenced by up-regulation of PERK-eIF2α-ATF4-CHOP. Chrysin treatment blocked such ER stress responses pertinent to podocyte apoptosis and reduced synthesis of slit diaphragm proteins in vitro and in vivo. These observations demonstrate that targeting ER stress is an underlying mechanism of chrysin-mediated amelioration of diabetes-associated podocyte injury and dysfunction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, Navarro-González JF . Diabetic kidney disease: from physiology to therapeutics. J Physiol 2014; 592: 3997–4012.
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008; 233: 4–11.
Garg P, Rabelink T . Glomerular proteinuria: a complex interplay between unique players. Adv Chronic Kidney Dis 2011; 18: 233–42.
Reddy GR, Kotlyarevska K, Ransom RF, Menon RK . The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hyperten 2008; 17: 32–6.
Diez-Sampedro A, Lenz O, Fornoni A . Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis 2011; 58: 637–46.
Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 2009; 58: 1201–11.
Wolf G, Chen S, Ziyadeh FN . From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005; 54: 1626–34.
Bhatti AB, Usman M . Drug targets for oxidative podocyte injury in diabetic nephropathy. Cureus 2015; 7: e393.
Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1237–54.
Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One 2013; 8: e60546.
Yasuda-Yamahara M, Kume S, Tagawa A, Maegawa H, Uzu T . Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy 2015; 11: 2385–6.
Cunard R . Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med 2015; 4: 715–40.
Taniguchi M, Yoshida H . Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hyperten 2015; 24: 345–50.
Cybulsky AV . The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 2013; 84: 25–33.
Woehlbier U, Hetz C . Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci 2011; 36: 329–37.
Yuan Y, Xu X, Zhao C, Zhao M, Wang H, Zhang B, et al. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone /mineralocorticoid receptor-induced podocyte injury. Lab Invest 2015; 95:1374–86.
Niforou K, Cheimonidou C, Trougakos IP . Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol 2014; 2: 323–32.
Rivas A, Vidal RL, Hetz C . Targeting the unfolded protein response for disease intervention. Expert Opin Therapeutic Targets 2015; 19: 1203–18.
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E . Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 2014; 728: 107–18.
Rashid S, Ali N, Nafees S, Hasan SK, Sultana S . Mitigation of 5-fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem Toxicol 2014; 66: 185–93.
Ali BH, Adham SA, Al Za'abi M, Waly MI, Yasin J, Nemmar A, et al. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS One 2015; 10: e0125285.
Kang MK, Park, SH, Choi YJ, Shin D, Kang YH . Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med (Berlin) 2015; 93: 759–72.
Lewko B, Stepinski J . Hyperglycemia and mechanical stress: targeting the renal podocyte. J Cell Physiol 2009; 221: 288–95.
Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, Schindler CK, et al. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ 2001; 8: 1169–81.
Ow YP, Green DR, Hao Z, Mak TW, Gao F . Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 2008; 9: 532–42.
Wang L, Tang Y, Eisner W, Sparks MA, Buckley AF, Spurney RF . Augmenting podocyte injury promotes advanced diabetic kidney disease in Akita mice. Biochem Biophys Res Commun 2014; 444: 622–7.
Zoja C, Abbate M, Remuzzi G . Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dialysis Transplant 2015; 30: 706–12.
Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y . The role of podocytes in proteinuria. Nephrology 2007; 12: S15–S20.
Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthöfer H, Zaoui P, et al. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 2002; 45: 1572–6.
Cybulsky AV, Takano T, Papillon J, Kitzler TM, Bijian K . Endoplasmic reticulum stress in glomerular epithelial cell injury. Am J Physiol Renal Physiol 2011; 301: F496–F508.
Mathieson PW . The podocyte as a target for therapies--new and old. Nat Rev Nephrol 2011; 8: 52–6.
Schönenberger E, Ehrich JH, Haller H, Schiffer M . The podocyte as a direct target of immunosuppressive agents. Nephrol Dialysis Transplant 2011; 26: 18–24.
Bao L, Cai X, Dai X, Ding Y, Jiang Y, Li Y, et al. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct 2014; 5: 1872–80.
Sun LN, Liu XC, Chen XJ, Guan GJ, Liu G . Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. Acta Pharmacol Sin 2016; 37: 645–55.
Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P . The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol 2013; 305: F691–F700.
Wang ZS, Xiong F, Xie, XH, Chen D, Pan JH, Cheng L . Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol 2015; 16: 44.
Brinkkoetter PT, Ising C, Benzing T . The role of the podocyte in albumin filtration. Nat Rev Nephrol 2013; 9: 328–36.
Pavenstädt H, Kriz W, Kretzler M . Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307.
DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M . Phosphorylation of eukaryotic translation initiation factor 2α coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol 2009; 29: 4295–307.
Dodson M, Darley-Usmar V, Zhang J . Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med 2013; 63: 207–21.
Oliva Trejo JA, Asanuma K, Kim EH, Takagi-Akiba M, Nonaka K, Hidaka T, et al. Transient increase in proteinuria, poly-ubiquitylated proteins and ER stress markers in podocyte-specific autophagy-deficient mice following unilateral nephrectomy. Biochem Biophys Res Commun 2014; 446: 1190–6.
Acknowledgements
This work was supported a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No 2015R1A2A2A01006666).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, MK., Park, SH., Kim, YH. et al. Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacol Sin 38, 1129–1140 (2017). https://doi.org/10.1038/aps.2017.30
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.30
Keywords
This article is cited by
-
Astragaloside IV Alleviates Podocyte Injury in Diabetic Nephropathy through Regulating IRE-1α/NF-κ B/NLRP3 Pathway
Chinese Journal of Integrative Medicine (2025)
-
Importance of unfolded protein response modulation on diabetes management: a systematic review
Journal of Diabetes & Metabolic Disorders (2024)
-
Natural Remedies and Health; A Review of Bee Pollen and Bee Bread Impact on Combating Diabetes and Obesity
Current Nutrition Reports (2024)
-
The effects of propolis on pro-oxidant–antioxidant balance, glycemic control, and quality of life in chronic kidney disease: a randomized, double-blind, placebo-controlled trial
Scientific Reports (2023)
-
Research progress on endoplasmic reticulum homeostasis in kidney diseases
Cell Death & Disease (2023)