Abstract
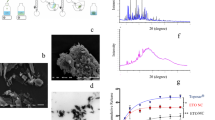
One of the major goals of precision oncology is to promote combination therapy to improve efficacy and reduce side effects of anti-cancer drugs based on their molecular mechanisms. In this study, we aimed to develop and validate new nanoformulations of docetaxel (DTX) and bortezomib (BTZ) for targeted combination therapy to treat human esophageal cancer. By leveraging our versatile disulfide cross-linked micelles (DCMs) platform, we developed nanoformulations of DTX and BTZ (named DTX-DCMs and BTZ-DCMs). Their physical properties were characterized; their anti-cancer efficacies and mechanisms of action were investigated in a human esophageal cancer cell line in vitro. Furthermore, the in vitro anti-tumor activities of combination therapies (concurrent drug treatment, sequential drug treatment, and treatment using different ratios of the drugs) were examined in comparison with the single drug treatment and free drug strategies. These drug-loaded nanoparticles were spherical in shape and relatively small in size of approximately 20–22 nm. The entrapment efficiencies of DTX and BTZ into nanoparticles were 82.4% and 84.1%, respectively. The drug release rates of DTX-DCMs and BTZ-DCMs were sustained, and greatly increased in the presence of GSH. These nanodrugs were effectively internalized by KYSE30 esophageal cancer cells, and dose-dependently induced cell apoptosis. We further revealed a strong synergistic effect between DTX-DCMs and BTZ-DCMs against KYSE30 esophageal cancer cells. Sequential combination therapy with DTX-DCMs followed by BTZ-DCMs exhibited the best anti-tumor efficacy in vitro. This study demonstrates that DTX and BTZ could be successfully nanoformulated into disulfide cross-linked micelles. The nanoformulations of DTX and BTZ demonstrate an immense potential for synergistic combination therapy to treat human esophageal cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32.
Collins DC, Sundar R, Lim JS, Yap TA . Towards precision medicine in the clinic: From biomarker discovery to novel therapeutics. Trends Pharmacol Sci 2017; 38: 25–40.
Tan Q, Liu X, Fu X, Li Q, Dou J, Zhai G . Current development in nanoformulations of docetaxel. Expert Opin Drug Deliv 2012; 9: 975–90.
Fushida S, Nashimoto A, Fukushima N, Kawachi Y, Fujimura T, Kuwabara S, et al. Phase II trial of preoperative chemotherapy with docetaxel, cisplatin and S-1 for T4 locally advanced gastric cancer. Jpn J Clin Oncol 2012; 42: 131–3.
Fei F, Chen C, Xue J, Di GH, Lu JS, Liu GY, et al. Efficacy and safety of docetaxel combined with oxaliplatin as a neoadjuvant chemotherapy regimen for Chinese triple-negative local advanced breast cancer patients. A prospective, open, and unicentric Phase II clinical trial. Am J Clin Oncol 2013; 36: 545–51.
Francis P, Schneider J, Hann L, Balmaceda C, Barakat R, Phillips M, et al. Phase II trial of docetaxel in patients with platinum-refractory advanced ovarian cancer. J Clin Oncol 1994; 12: 2301–8.
Hironaka S, Tsubosa Y, Mizusawa J, Kii T, Kato K, Tsushima T, et al. Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci 2014; 105: 1189–95.
Des Guetz G, Landre T, Westeel V, Milleron B, Vaylet F, Urban T, et al. Similar survival rates with first-line gefitinib, gemcitabine, or docetaxel in a randomized phase II trial in elderly patients with advanced non-small cell lung cancer and a poor performance status (IFCT-0301). J Geriatr Oncol 2015; 6: 233–40.
Baker SD, Sparreboom A, Verweij J . Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet 2006; 45: 235–52.
Clarke SJ, Rivory LP . Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 1999; 36: 99–114.
Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 2001; 61: 3071–6.
You SA, Basu A, Haldar S . Potent antitumor agent proteasome inhibitors: a novel trigger for Bcl2 phosphorylation to induce apoptosis. Int J Oncol 1999; 15: 625–8.
Ocean AJ, Christos P, Sparano JA, Shah MA, Yantiss RK, Cheng J, et al. Phase II trial of bortezomib alone or in combination with irinotecan in patients with adenocarcinoma of the gastroesophageal junction or stomach. Invest New Drugs 2014; 32: 542–8.
Nawrocki ST, Sweeney-Gotsch B, Takamori R, McConkey DJ . The proteasome inhibitor bortezomib enhances the activity of docetaxel in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther 2004; 3: 59–70.
Canfield SE, Zhu K, Williams SA, McConkey DJ . Bortezomib inhibits docetaxel-induced apoptosis via a p21-dependent mechanism in human prostate cancer cells. Mol Cancer Ther 2006; 5: 2043–50.
Chung CH, Aulino J, Muldowney NJ, Hatakeyama H, Baumann J, Burkey B, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2010; 21: 864–70.
Lara PN Jr, Longmate J, Reckamp K, Gitlitz B, Argiris A, Ramalingam S, et al. Randomized phase II trial of concurrent versus sequential bortezomib plus docetaxel in advanced non-small-cell lung cancer: a California cancer consortium trial. Clin Lung Cancer 2011; 12: 33–7.
Awada A, Albanell J, Canney PA, Dirix LY, Gil T, Cardoso F, et al. Bortezomib/docetaxel combination therapy in patients with anthracycline-pretreated advanced/metastatic breast cancer: a phase I/II dose-escalation study. Br J Cancer 2008; 98: 1500–7.
Zhang M, Wei W, Liu J, Yang H, Jiang Y, Tang W, et al. Comparison of the effectiveness and toxicity of neoadjuvant chemotherapy regimens, capecitabine/epirubicin/cyclophosphamide vs 5-fluorouracil/epirubicin/cyclophosphamide, followed by adjuvant, capecitabine/docetaxel vs docetaxel, in patients with operable breast cancer. Onco Targets Ther 2016; 9: 3443–50.
Schweizer MT, Gulati R, Mostaghel EA, Nelson PS, Montgomery RB, Yu EY, et al. Docetaxel-related toxicity in metastatic hormone-sensitive and metastatic castration-resistant prostate cancer. Med Oncol 2016; 33: 77.
Bruchim I, Weeg N, Alpert Y, Sade D, Piura E, Fishman A . High efficacy and low toxicity of the modified docetaxel and carboplatin protocol in patients with recurrent ovarian cancer-a phase 2 cohort study. Int J Gynecol Cancer 2016; 26: 640–7.
Chanat C, Delbaldo C, Denis J, Bocaccio F, Cojean-Zelek I, Le Guyader N . Dose intensity and toxicity associated with Taxotere formulation: a retrospective study in a population of breast cancer patients treated with docetaxel as an adjuvant or neoadjuvant chemotherapy. Anticancer Drugs 2015; 26: 984–9.
Senapati PC, Sahoo SK, Sahu AN . Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed Pharmacother 2016; 80: 42–51.
Gao Y, Shen JK, Choy E, Zhang Z, Mankin HJ, Hornicek FJ, et al. Pharmacokinetics and tolerability of NSC23925b, a novel P-glycoprotein inhibitor: preclinical study in mice and rats. Sci Rep 2016; 6: 25659.
Koo AN, Lee HJ, Kim SE, Chang JH, Park C, Kim C, et al. Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem Commun (Camb) 2008; (48): 6570–2.
Yu S, Ding J, He C, Cao Y, Xu W, Chen X . Disulfide cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy. Adv Healthc Mater 2014; 3: 752–60.
Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials 2011; 32: 6633–45.
Ho MY, Mackey JR . Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag Res 2014; 6: 253–9.
Xiao K, Li YP, Wang C, Ahmad S, Vu M, Kuma K, et al. Disulfide cross-linked micelles of novel HDAC inhibitor thailandepsin A for the treatment of breast cancer. Biomaterials 2015; 67: 183–93.
Vysloužil J, Bavoľárová J, Kejdušová M, Vetchý D, Dvořáčková K . Cationic Eudragit® polymers as excipients for microparticles prepared by solvent evaporation method. Ceska Slov Farm 2013; 62: 249–54.
Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, et al. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem 2010; 21: 1216–24.
Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 2009; 30: 6006–16.
Xiao K, Luo J, Li Y, Lee J S, Fung G, Lam KS . PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-cell lymphoma. J Control Release 2011; 155: 272–81.
Wei X, Luo Q, Sun L, Li X, Zhu H, Guan P, et al. Enzyme- and pH-sensitive branched polymer-doxorubicin conjugate-based nanoscale drug delivery system for cancer therapy. ACS Appl Mater Interfaces 2016; 8: 11765–78.
An J, Dai X, Wu Z, Zhao Y, Lu Z, Guo Q, et al. An acid-triggered degradable and fluorescent nanoscale drug delivery system with enhanced cytotoxicity to cancer cells. Biomacromolecules 2015; 16: 2444–54.
Brown PK, Qureshi AT, Moll AN, Hayes DJ, Monroe WT . Silver nanoscale antisense drug delivery system for photoactivated gene silencing. ACS Nano 2013; 7: 2948–59.
Yoshida H, Kim YH, Ozasa H, Nagai H, Sakamori Y, Nakaoku T, et al. Albumin-bound paclitaxel for the treatment of refractory or relapsed small-cell lung cancer. Mol Clin Oncol 2016; 5: 213–5.
Rohlfing S, Aurich M, Schöning T, Ho AD, Witzens-Harig M . Nonpegylated liposomal doxorubicin as a component of R-CHOP is an effective and safe alternative to conventional doxorubicin in the treatment of patients with diffuse large B-Cell lymphoma and preexisting cardiac diseases. Clin Lymphoma Myeloma Leuk 2015; 15: 458–63.
Park IH, Sohn JH, Kim SB, Lee KS, Chung JS, Lee SH, et al. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res Treat 2016. doi: 10.4143/crt.2016.289.
Yokoyama M . Drug targeting with nano-sized carrier systems. J Artif Organs 2005; 8: 77–84.
Xiong F, Tian J, Hu K, Zheng X, Sun J, Yan C, et al. Superparamagnetic anisotropic nano-assemblies with longer blood circulation in vivo: a highly efficient drug delivery carrier for leukemia therapy. Nanoscale 2016; 8: 17085–9.
Kohli AK, Alpar HO . Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm 2004; 275: 13–7.
Boutros T, Chevet E, Metrakos P . Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 2008; 60: 261–310.
Chauvin L, Goupille C, Blanc C, Pinault M, Domingo I, Guimaraes C, et al. Long chain n-3 polyunsaturated fatty acids increase the efficacy of docetaxel in mammary cancer cells by downregulating Akt and PKCɛ/δ-induced ERK pathways. Biochim Biophys Acta 2016; 1861: 380–90.
McDaid HM, Lopez-Barcons L, Grossman A, Lia M, Keller S, Pérez-Soler R, et al. Enhancement of the therapeutic efficacy of taxol by the mitogen-activated protein kinase kinase inhibitor CI-1040 in nude mice bearing human heterotransplants. Cancer Res 2005; 65: 2854–60.
Panday A, Inda ME, Bagam P, Sahoo MK, Osorio D, Batra S . Transcription factor NF-κB: an update on intervention strategies. Arch Immunol Ther Exp (Warsz) 2016; 64: 463–83.
Yang G, Xiao X, Rosen DG, Cheng X, Wu X, Chang B, et al. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res 2011; 17: 2181–94.
Acknowledgements
This work was financially supported by NIH/NCI (R01CA199668) and NIH/NICHD (R01HD086195).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Xs., Kong, Dj., Lin, Ty. et al. A versatile nanoplatform for synergistic combination therapy to treat human esophageal cancer. Acta Pharmacol Sin 38, 931–942 (2017). https://doi.org/10.1038/aps.2017.43
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.43
Keywords
This article is cited by
-
Cancer nanobiotechnolgy
Acta Pharmacologica Sinica (2017)