Abstract
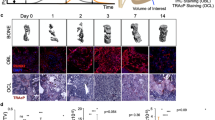
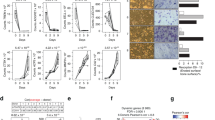
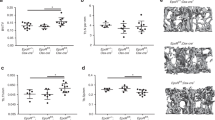
Osteoporotic treatments have largely depended on antiresorptive or anabolic drugs; but the former also suppresses new bone formation, and the latter only includes human parathyroid hormone. There is no drug that has a dual effect to inhibit bone resorption and to stimulate bone formation simultaneously. Here, we report a small molecule, a quinoxaline derivative of oleanolic acid (QOA-8a) that plays such dual roles in osteoblasts and osteoclasts in the treatment of osteoporosis. Osteoclast differentiation was induced by incubation of primary mouse bone marrow-derived macrophages in the presence of RANKL and M-CSF, treatment with QOA-8a dose-dependently inhibited the osteoclast formation with an IC50 value of 0.098 μmol/L. QOA-8a also directly acted on osteoblasts, and stimulated new bone formation in murine calvarial bones in vitro and in vivo. In an OVX rat model, administration of QOA-8a (1, 5 mg·kg−1·d−1, po) for 16 weeks effectively prevented OVX-induced bone loss, accompanied by decreased serum levels of the bone resorption marker CTX-1 and increased serum levels of osteoblast marker N-MID-OT. Meaningfully, our preliminary study revealed that QOA-8a down-regulated the ERK1/2 signal in osteoclasts and up-regulated the signal in osteoblasts. QOA-8a showed dual functions in both animal and human osteoclastogenesis and osteoblastogenesis. Our results demonstrate that QOA-8a might serve as a lead compound with a dual function of bone anabolic and anti-resorptive effects in the development of anti-osteoporosis agents.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aw D, Thain J, Ali A, Aung T, Chua WM, Sahota O, et al. Predicting fracture risk in osteoporosis: the use of fracture prediction tools in an osteoporosis clinic population. Postgrad Med J 2016; 92: 267–70.
Hofbauer LC, Rachner TD . WNT signaling in bone homeostasis and disease: from human mutations to treatments. Lancet 2015; 386: 1116–8.
Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P . Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 2010; 8: 1–16.
Johnell O, Kanis JA . An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004; 15: 897–902.
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY . European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013; 24: 23–57.
Dempster DW . Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care 2011; 17: S164–169.
General Office of the Ministry of Health of the People's Republic of China. Outline of prevention and treatment of osteoporosis. Capital J Public Health 2011; 5: 146–9.
Harsløf T, Langdahl BL . New horizons in osteoporosis therapies. Curr Opin Pharmacol 2016; 28: 38–42.
Opar A . Late-stage osteoporosis drugs illustrate challenges in the field. Nat Rev Drug Discov 2009; 8: 757–8.
McClung MR . Emerging therapies for osteoporosis. Endocrinol Metab 2015; 30: 429–35.
Deal C . Potential new drug targets for osteoporosis. Nat Rev Rheumatol 2009; 5: 20–7.
Garber K . Two pioneering osteoporosis drugs finally approach approval. Nat Rev Drug Discov 2016; 15: 445–6.
Deeks ED, Dhillon S . Spotlight on strontium ranelate in post-menopausal osteoporosis. Drugs Aging 2010; 27: 771–3.
Ma YFL, Zeng QQ, Porras LL, Harvey A . Moore TL, Shelbourn TL, et al. Teriparatide [rhPTH (1–34)], but not strontium ranelate, demonstrated bone anabolic efficacy in mature, osteopenic, ovariectomized rats. Endocrinology 2011; 152: 1767–78.
Fuchs RK, Allen MR, Condon KW, Reinwald S, Miller LM, McClenathan D, et al. Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporos Int 2008; 19: 1331–41.
Gupta A, March L . Treating osteoporosis. Aust Prescr 2016; 39: 40–6.
Baron R, Ferrari S, Russell RG . Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48: 677–92.
Koch L . Therapy: Raising BMD—two drugs are better than one. Nat Rev Endocrinol 2013; 9: 442.
Herrero S, Pico Y . Treatments for post-menopausal osteoporotic women, what's new? How can we manage long-term treatment? J Eur J Pharmacol 2016; 779: 8–21.
Ilić D, Bukumirić Z, Janković S . Drug-related problems in patients with osteoporosis. Vojnosanit Pregl 2016; 73: 261–5.
Newman DJ, Cragg GM . Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 2012; 75: 311–35.
Li JX, Hareyama T, Tezuka Y, Zhang Y, Miyahara T, Kadota S . Five new oleanolic acid glycosides from Achyranthes bidentata with inhibitory activity on osteoclast formation. Planta Med 2005; 71: 673–9.
He CC, Hui RR, Tezuka Y, Kadota K, Li JX . Osteoprotective effect of extract from Achyranthes bidentata in ovariectomized rats. J Ethnopharmacol 2010; 127: 229–34.
Li JF, Zhao Y, Cai MM, Li XF, Li JX . Synthesis and evaluation of a novel series of heterocyclic oleanolic acid derivatives with anti-osteoclast formation activity. Eur J Med Chem 2009; 44: 2796–806.
Zhao Y, Huai Y, Jin J, Geng M, Li JX . Quinoxaline derivative of oleanolic acid (QOA-8a) inhibits osteocalstic bone resorption and prevents ovariectomy induced bone loss. Menopause 2011; 18: 690–7.
Nadri S, Soleimani M, Hosseni RH, Massumi, M, Atashi A, Izadpanah R . An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol 2007; 51: 723–9.
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 286: 1946–9.
Clines GA, Mohammad KS, Bao YD, Stephens OW, Suva LJ, Shaughnessy JD, et al. Dickkopf homolog 1 mediates endothelin-1-stimulated new bone formation. Mol Endocrinol 2007; 21: 486–98.
Katy JLB, Andrew H, Les I, Marc C H, Kristine EE, Steven R, et al. The Potential value of monitoring bone turnover markers among women on alendronate. J Bone Miner Res 2012; 27: 195–201.
Kharkwal G, Chandra V, Fatima I, Dwivedi A . Ormeloxifene inhibits osteoclast differentiation in parallel to downregulating RANKL-induced ROS generation and suppressing the activation of ERK and JNK in murine RAW264.7 cells. J Mol Endocrinol 2012; 48: 261–70.
Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL regulated osteoclast differentiation. J Clin Invest 2004; 114: 475–84.
Robling AG, Castillo AB, Turner CH . Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 2006; 8: 455–98.
Tian XY, Jee WSS, Li XD, Paszty C, Ke HZ . Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 2011; 48: 197–201.
Boyle WJ, Simonet WS, Lacey DL . Osteoclast differentiation and activation. Nature 2003; 423: 337–42.
Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 2005; 202: 589–95.
Novack DV . Role of NF-κB in the skeleton. Cell Res 2011; 21: 169–82.
Wei ZF, Tong B, Xia YF, Lu Q, Chou GX, Wang ZT, et al. Norisoboldine suppresses osteoclast differentiation through preventing the accumulation of TRAF6-TAK1 complexes and activation of MAPKs/NF-kB/c-Fos/NFATc1 pathways. PLoS One 2013; 8: e59171.
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994; 266: 443–8.
Boyce BF, Yao ZQ, Xing LP . Functions of nuclear factor κB in bone. Ann NY Acad Sci 2010; 1192: 367–75.
Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV . RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest 2008; 118: 2088–97.
Li JL . JAK-STAT and bone metabolism. JAK-STAT 2013; 2: e23930.
Zhou H, Newnum AB, Martin JR, Li P, Nelson MT, Moh A, et al. Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone 2011; 49: 404–11.
Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 2006; 38: 1424–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 21272114, 90913023). We thank the Shanghai Synchrotron Radiation Facility (SSRF) for the microcomputed tomography (μCT) analysis of bone. We thank Prof Shigetoshi KADOTA and Dr Feng LI from the University of Toyama, Japan, for the peripheral quantitative computed tomography (pQCT) analysis of bone.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Shen, Q., Cui, W. et al. Dual roles of QOA-8a in antiosteoporosis: a combination of bone anabolic and anti-resorptive effects. Acta Pharmacol Sin 39, 230–242 (2018). https://doi.org/10.1038/aps.2017.63
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.63