Abstract
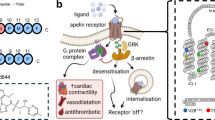
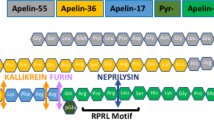
Apelin is the endogenous ligand for the G protein-coupled receptor APJ, and plays important roles in the cardiovascular system. Our previous studies showed that apelin-13 promotes the hypertrophy of H9c2 rat cardiomyocytes through the PI3K-autophagy pathway. The aim of this study was to explore what roles ER stress and autophagy played in apelin-13-induced hypertrophy of cardiomyocytes in vitro. Treatment of H9c2 cells with apelin-13 (0.001–2 μmol/L) dose-dependently increased the production of ROS and the expression levels of NADPH oxidase 4 (NOX4). Knockdown of Nox4 with siRNAs effectively prevented the reduction of GSH/GSSG ratio in apelin-13-treated cells. Furthermore, apelin-13 treatment dose-dependently increased the expression of Bip and CHOP, two ER stress markers, in the cells. Knockdown of APJ or Nox4 with the corresponding siRNAs, or application of NADPH inhibitor DPI blocked apelin-13-induced increases in Bip and CHOP expression. Moreover, apelin-13 treatment increased the formation of autophagosome and ER fragments and the LC3 puncta in the ER of the cells. Knockdown of APJ, Nox4, Bip or CHOP with the corresponding siRNAs, or application of DPI or salubrinal attenuated apelin-13-induced overexpression of LC3-II/I and beclin 1. Finally, knockdown of Nox4, Bip or CHOP with the corresponding siRNAs, or application of salubrinal significantly suppressed apelin-13-induced increases in the cell diameter, volume and protein contents. Our results demonstrate that ER stress-autophagy is involved in apelin-13-induced H9c2 cell hypertrophy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol 2009; 297: H1904–13.
Falcao-Pires I, Goncalves N, Gavina C, Pinho S, Teixeira T, Moura C, et al. Correlation between plasma levels of apelin and myocardial hypertrophy in rats and humans: possible target for treatment? Expert Opin Ther Targets 2010; 14: 231–41.
Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature 2012; 488: 394–8.
XIE F, Li LF, CHEN LX . APJ act as a reponse for pressure overload to induce myocardial hypertrophy. Prog Biochem Biophys 2013; 40: 33–6.
Li L, Zeng H, Chen JX . Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol 2012; 303: H605–18.
Xie F, Liu W, Feng F, Li X, Yang L, Lv D, et al. A static pressure sensitive receptor APJ promote H9c2 cardiomyocyte hypertrophy via PI3K-autophagy pathway. Acta Biochim Biophys Sin (Shanghai) 2014; 46: 699–708.
Guan HS, Shangguan HJ, Shang Z, Yang L, Meng XM, Qiao SB . Endoplasmic reticulum stress caused by left ventricular hypertrophy in rats: effects of telmisartan. Am J Med Sci 2011; 342: 318–23.
Cheng Y, Yang JM . Survival and death of endoplasmic-reticulum-stressed cells: Role of autophagy. World J Biol Chem 2011; 2: 226–31.
Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J . Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 2011; 106: 1173–91.
Gao L, Laude K, Cai H . Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract 2008; 38: 137–55.
Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 2003; 93: 802–5.
Wu RF, Ma Z, Liu Z, Terada LS . Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 2010; 30: 3553–68.
Schuck S, Gallagher CM, Walter P . ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J Cell Sci 2014; 127: 4078–88.
Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 2005; 65: 73–82.
Li L, Li F, Li F, Mao X, Yang L, Guo Y, et al. NOX4-derived reactive oxygen species drive Apelin-13-induced vascular smooth muscle cell proliferation via the ERK pathway. Int J Pept Res Ther 2011; 17: 307–15.
Mao XH, Tao SU, Zhang XH, Fang L, Qin XP, Li X, et al. Apelin-13 promote monocytes adhesion to HUVECs via PI3K signaling. Prog Biochem Biophys 2011; 38: 1162–70.
Liu C, Su T, Li F, Li L, Qin X, Pan W, et al. PI3K/Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin-13. Acta Biochim Biophys Sin (Shanghai) 2010; 42: 396–402.
Pan WN, Feng L, Mao XH, Qin XP, Deng SX, Feng F, et al. 14-3-3 protein is involved in ERK1/2 signaling transduction pathway of rat vascular smooth muscle cells proliferation induced by apelin-13. Prog Biochem Biophys 2011; 38: 1153–61.
Li L, Li L, Xie F, Zhang Z, Guo Y, Tang G, et al. Jagged-1/Notch3 signaling transduction pathway is involved in apelin-13-induced vascular smooth muscle cells proliferation. Acta Biochim Biophys Sin (Shanghai) 2013; 45: 875–81.
Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol 2012; 302: C597–604.
Li X, Zhang X, Li F, Chen L, Li L, Qin X, et al. 14-3-3 mediates apelin-13-induced enhancement of adhesion of monocytes to human umbilical vein endothelial cells. Acta Biochim Biophys Sin (Shanghai) 2010; 42: 403–9.
Kleinz MJ, Davenport AP . Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 2004; 118: 119–25.
Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 2004; 110: II187–93.
Ni L, Zhou C, Duan Q, Lv J, Fu X, Xia Y, et al. beta-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS One 2011; 6: e27294.
Xie F, Li L, Chen L . Autophagy, a new target for disease treatment. Sci China Life Sci 2013; 56: 856–60.
Green DR, Levine B . To be or not to be? How selective autophagy and cell death govern cell fate. Cell 2014; 157: 65–75.
Cebollero E, Reggiori F, Kraft C . Reticulophagy and ribophagy: regulated degradation of protein production factories. Int J Cell Biol 2012; 2012: 182834.
Tasdemir E, Maiuri MC, Tajeddine N, Vitale I, Criollo A, Vicencio JM, et al. Cell cycle-dependent induction of autophagy, mitophagy and reticulophagy. Cell Cycle 2007; 6: 2263–7.
Kulich I, Zarsky V . Autophagy-related direct membrane import from ER/Cytoplasm into the vacuole or apoplast: a hidden gateway also for secondary metabolites and phytohormones? Int J Mol Sci 2014; 15: 7462–74.
Deegan S, Saveljeva S, Gorman AM, Samali A . Stress-induced self-cannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell Mol Life Sci 2013; 70: 2425–41.
Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, et al. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Hum Mol Genet 2012; 21: 2245–62.
Wang J, Kang R, Huang H, Xi X, Wang B, Wang J, et al. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy 2014; 10: 766–84.
Yang L, Zhao D, Ren J, Yang J . Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim Biophys Acta 2015; 1852: 209–18.
Zhang B, Zhang Y, La Cour KH, Richmond KL, Wang XM, Ren J . Mitochondrial aldehyde dehydrogenase obliterates endoplasmic reticulum stress-induced cardiac contractile dysfunction via correction of autophagy. Biochim Biophys Acta 2013; 1832: 574–84.
Lv S, Sun EC, Xu QY, Zhang JK, Wu DL . Endoplasmic reticulum stress-mediated autophagy contributes to bluetongue virus infection via the PERK-eIF2α pathway. Biochem Biophys Res Commun 2015; 466: 406–12.
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 2007; 14: 230–9.
Sasaya H, Utsumi T, Shimoke K, Nakayama H, Matsumura Y, Fukunaga K, et al. Nicotine suppresses tunicamycin-induced, but not thapsigargin-induced, expression of GRP78 during ER stress-mediated apoptosis in PC12 cells. J Biochem 2008; 144: 251–7.
Bull VH, Thiede B . Proteome analysis of tunicamycin-induced ER stress. Electrophoresis 2012; 33: 1814–23.
Wen X, Wu J, Wang F, Liu B, Huang C, Wei Y . Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic Biol Med 2013; 65: 402–10.
Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, et al. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012; 302: L651–63.
Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, et al. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A 2009; 106: 6226–31.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81503074, 81270420, 81470434, and 81670265), Hunan Provincial Natural Science Foundation (14JJ3102; 2017JJ2227), China Postdoctoral Science Foundation (2014M560647). We especially thank Bing-bing WANG (Assistant Professor, Perinatal Biology Laboratory, Division of Maternal-Fetal Medicine, Rutgers University-Robert Wood Johnson Medical School, USA) for the English language correction.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, F., Wu, D., Huang, Sf. et al. The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy in vitro. Acta Pharmacol Sin 38, 1589–1600 (2017). https://doi.org/10.1038/aps.2017.97
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2017.97
Keywords
This article is cited by
-
SEC62-dependent ER-phagy contributes to apelin-13/APJ-induced monocyte-vascular endothelial cell adhesion in atherosclerosis pathogenesis
Acta Pharmacologica Sinica (2025)
-
Beyond conventional therapy: NOX4 as a promising target in cardiomyopathy
Molecular Biology Reports (2025)
-
HTRA1-driven detachment of type I collagen from endoplasmic reticulum contributes to myocardial fibrosis in dilated cardiomyopathy
Journal of Translational Medicine (2024)
-
Chronic infusion of ELABELA alleviates vascular remodeling in spontaneously hypertensive rats via anti-inflammatory, anti-oxidative and anti-proliferative effects
Acta Pharmacologica Sinica (2022)
-
STING protects against cardiac dysfunction and remodelling by blocking autophagy
Cell Communication and Signaling (2021)