Abstract
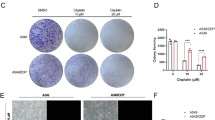
NF-E2-related factor 2 (Nrf2) is a transcription factor and a pivotal factor in the induction of the cell defense system. Recent reports show that Nrf2 plays critical roles in tumor heterogeneity and drug resistance. In the present study we investigated whether and how Nrf2 mediated the resistance of human cancer cells to boningmycin (BON), a new antitumor antibiotic of the bleomycin family. We showed that in the expression levels of Nrf2 in human non-small lung cancer A549 cells were much higher than those in human hepatoblastoma HepG2 cells, and their resistance to BON was opposite to Nrf2 expression (the IC50values of BON in A549 cells and HepG2 cells were 5.97 and 0.61 μmol/L, respectively). Similar results were observed with the anticancer agent cisdiamminedichloroplatinum (DDP), which was used as a positive control. In A549 cells, Nrf2 mRNA knockdown significantly increased their susceptibilities to BON and DDP. An enhanced resistance to BON and DDP was observed in HepG2 cells after overexpression of the wild-type Nrf2 protein. Treatment with a specific Nrf2 inhibitor, luteolin, significantly sensitized A549 cells to BON and DDP and increased BON- or DDP-induced apoptosis. The total levels of glutathione (GSH), the final product of the Nrf2 signaling pathway, were much higher in A549 cells than those in HepG2 cells. Supplementation of GSH in HepG2 cells significantly decreased their susceptibility to BON and DDP, wheras depleting GSH with the specific inhibitor L-buthionine sulfoximine in A549 cells significantly increased their susceptibility to BON and DDP. Our results demonstrate that Nrf2 mediates the resistance to BON through regulating glutathione levels in A549 cells and HepG2 cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Xu H, Yu L, Zhang X, Wang S. Isolation, purification and structure determination of boningmycin (Z-893). J Chin Antibiot 2003; 28: 465–7.
Gao N, Shang B, Zhang X, Shen C, Xu R, Xu H, et al. Potent antitumor actions of the new antibiotic boningmycin through induction of apoptosis and cellular senescence. Anticancer Drugs 2011; 22: 166–75.
Chen J, Chen Y, He Q. Action of bleomycin is affected by bleomycin hydrolase but not by caveolin-1. Int J Oncol 2012; 41: 2245–52.
Schwartz DR, Homanics GE, Hoyt DG, Klein E, Abernethy J, Lazo JS. The neutral cysteine protease bleomycin hydrolase is essential for epidermal integrity and bleomycin resistance. Proc Natl Acad Sci U S A 1999; 96: 4680–5.
Yen HC, Li SH, Majima HJ, Huang YH, Chen CP, Liu CC, et al. Up-regulation of antioxidant enzymes and coenzyme Q(10) in a human oral cancer cell line with acquired bleomycin resistance. Free Radic Res 2011; 45: 707–16.
Aouida M, Poulin R, Ramotar D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J Biol Chem 2010; 285: 6275–84.
Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer 2005; 5: 102–12.
Sanz G, Mir L, Jacquemin-Sablon A. Bleomycin resistance in mammalian cells expressing a genetic suppressor element derived from the SRPK1 gene. Cancer Res 2002; 62: 4453–8.
Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 2013; 34: 340–6.
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010; 12: 213–23.
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 2009; 34: 663–73.
Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 2006; 281: 39776–84.
Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med 2015; 88: 93–100.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475: 106–9.
Tao S, Wang S, Moghaddam SJ, Ooi A, Chapman E, Wong PK, et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res 2014; 74: 7430–41.
Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res 2009; 15: 3423–32.
Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 2008; 68:7975–84.
Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal 2010; 13: 1627–37.
Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 2011; 108: 1433–8.
Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, et al. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med 2011; 50: 1599–609.
Olayanju A, Copple IM, Bryan HK, Edge GT, Sison RL, Wong MW, et al. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic Biol Med 2015; 78: 202–12.
Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res 2010; 11: 31.
Pujari G, Sarma A, Chatterjee A. The influence of reduced glutathione on chromosome damage induced by X-rays or heavy ion beams of different LETs and on the interaction of DNA lesions induced by radiations and bleomycin. Mutat Res 2010; 696: 154–9.
Pujari G, Berni A, Palitti F, Chatterjee A. Influence of glutathione levels on radiation-induced chromosomal DNA damage and repair in human peripheral lymphocytes. Mutat Res 2009; 675: 23–8.
Chen Y, Xu R, Chen J, Li X, He Q. Cleavage of bleomycin hydrolase by caspase-3 during apoptosis. Oncol Rep 2013; 30: 939–44.
Lu SC. Glutathione synthesis. Biochim Biophys Acta 2013; 1830: 3143–53.
Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A 2002; 99: 4592–5.
Hanna NH, Einhorn LH. Testicular cancer-discoveries and updates. N Engl J Med 2014; 371: 2005–16.
Burgy O, Wettstein G, Bellaye PS, Decologne N, Racoeur C, Goirand F, et al. Deglycosylated bleomycin has the antitumor activity of bleomycin without pulmonary toxicity. Sci Transl Med 2016; 8: 326ra20.
Simpson AB, Paul J, Graham J, Kaye SB. Fatal bleomycin pulmonary toxicity in the west of Scotland 1991-95: a review of patients with germ cell tumours. Br J Cancer 1998; 78: 1061–6.
de Haas EC, Zwart N, Meijer C, Nuver J, Boezen HM, Suurmeijer AJ, et al. Variation in bleomycin hydrolase gene is associated with reduced survival after chemotherapy for testicular germ cell cancer. J Clin Oncol 2008; 26: 1817–23.
Oshimori N, Oristian D, Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 2015; 160: 963–76.
Chio II, Jafarnejad SM, Ponz-Sarvise M, Park Y, Rivera K, Palm W, et al. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell 2016; 166: 963–76.
Tao S, Wang S, Moghaddam SJ, Ooi A, Chapman E, Wong PK, et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res 2014; 74: 7430–41.
Acknowledgements
The work was supported by grants from the National Natural Science Foundation of China (No 81273553 and 31471150).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure S1
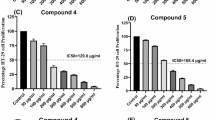
LUT reduced Nrf2 protein expression and showed almost no suppression of cell proliferation at 10 μmol/L in A549 cells.
Rights and permissions
About this article
Cite this article
Zhang, Hx., Chen, Y., Xu, R. et al. Nrf2 mediates the resistance of human A549 and HepG2 cancer cells to boningmycin, a new antitumor antibiotic, in vitro through regulation of glutathione levels. Acta Pharmacol Sin 39, 1661–1669 (2018). https://doi.org/10.1038/aps.2018.21
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2018.21